Naming compounds, Naming Chemical Compounds
magnesium nitrate
Mg(NO₃)₂
calcium hydride
CaH₂
1/100
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
101 Terms
magnesium nitrate
Mg(NO₃)₂
calcium hydride
CaH₂
copper(II) sulfide
CuS
potassium chromate
K₂CrO₄
aluminum acetate
Al(C₂H₃O₂)₃
nickel sulfate
NiSO₄
lead(II) sulfide
PbS
lead(II) chlorate
Pb(ClO₃)₂
manganese oxalate
MnC₂O₄
carbon tetrachloride
CCl₄
ammonium sulfate
(NH₄)₂SO₄
cobalt(II) hydroxide
Co(OH)₂
lithium perchlorate
LiClO₄
copper(II) sulfate
CuSO₄
zinc nitrite
Zn(NO₂)₂
rubidium hydride
RbH
barium nitride
Ba₃N₂
tetraphosphorus decoxide
P₄O₁₀
silver cyanide
AgCN
carbon disulfide
CS₂
cesium dihydrogen phosphate
CsH₂PO₄
rubidium chromate
Rb₂CrO₄
ammonium phosphate
(NH₄)₃PO₄
disulfur dichloride
S₂Cl₂
sodium hydrogen phosphate
Na₂HPO₄
tin(II) hydrogen carbonate
Sn(HCO₃)₂
chromium(III) acetate
Cr(C₂H₃O₂)₃
nickel carbonate
NiCO₃
aluminum oxalate
Al₂(C₄O₄)₃
KCN
potassium cyanide
titanium sulfite
Ti(SO₃)₂
sulfur trioxide
SO₃
lithium permanganate
LiMnO₄
SF₆
sulfur hexafluoride
dinitrogen pentoxide
N₂O₅
NO
nitrogen monoxide
CO
carbon monoxide
calcium hypochlorite
Ca(ClO)₂
magnesium hydroxide
Mg(OH)₂
CO₂
carbon dioxide
NO₂
nitrogen dioxide
ZnS
zinc sulfide
CuCl₂
copper(II) chloride
MgCl₂
magnesium chloride
Ca(ClO₃)₂
calcium chlorate
LiNO₂
lithium nitrite
CaSO₄
calcium sulfate
KH₂PO₄
potassium dihydrogen phosphate
AgNO₃
silver nitrate
CuCN
copper(I) cyanide
KHCO₃
potassium hydrogen carbonate
CaO
calcium oxide
NaHSO₄
sodium hydrogen sulfate
H₂CO₃
carbonic acid
Li₂HPO₄
lithium hydrogen phosphate
Mg₃(PO₄)₂
magnesium phosphate
KCl
potassium chloride
MgSO₄
magnesium sulfate
K₂O
potassium oxide
Al(NO₂)₃
aluminum nitrite
SiO₂
silicon dioxide
MgO
magnesium oxide
CuCl
copper(I) chloride
KClO₂
potassium chlorite
CaSO₃
calcium sulfite
CuSO₃
copper(II) sulfite
NaBr
sodium bromide
HF
hydrofluoric acid
FeSO₄
iron(II) sulfate
NaH
sodium hydride
NH₄OH
ammonium hydroxide
Pb(NO₃)₂
lead(II) nitrate
Fe(ClO₄)₃
iron(III) perchlorate
H₃PO₄
phosphoric acid
CS₂
carbon disulfide
CaH₂
calcium hydride
P₂O₃
diphosphorus trioxide
diphosphorus trioxide
P₂O₃
P₄O₁₀
tetraphosphorus decoxide
iodine monochloride
ICl
Cations
any atom or group of atoms that has a positive negative charge

Anions
atoms or group of atoms that have a negative charge
Ionic Compounds
composed of cation and anions
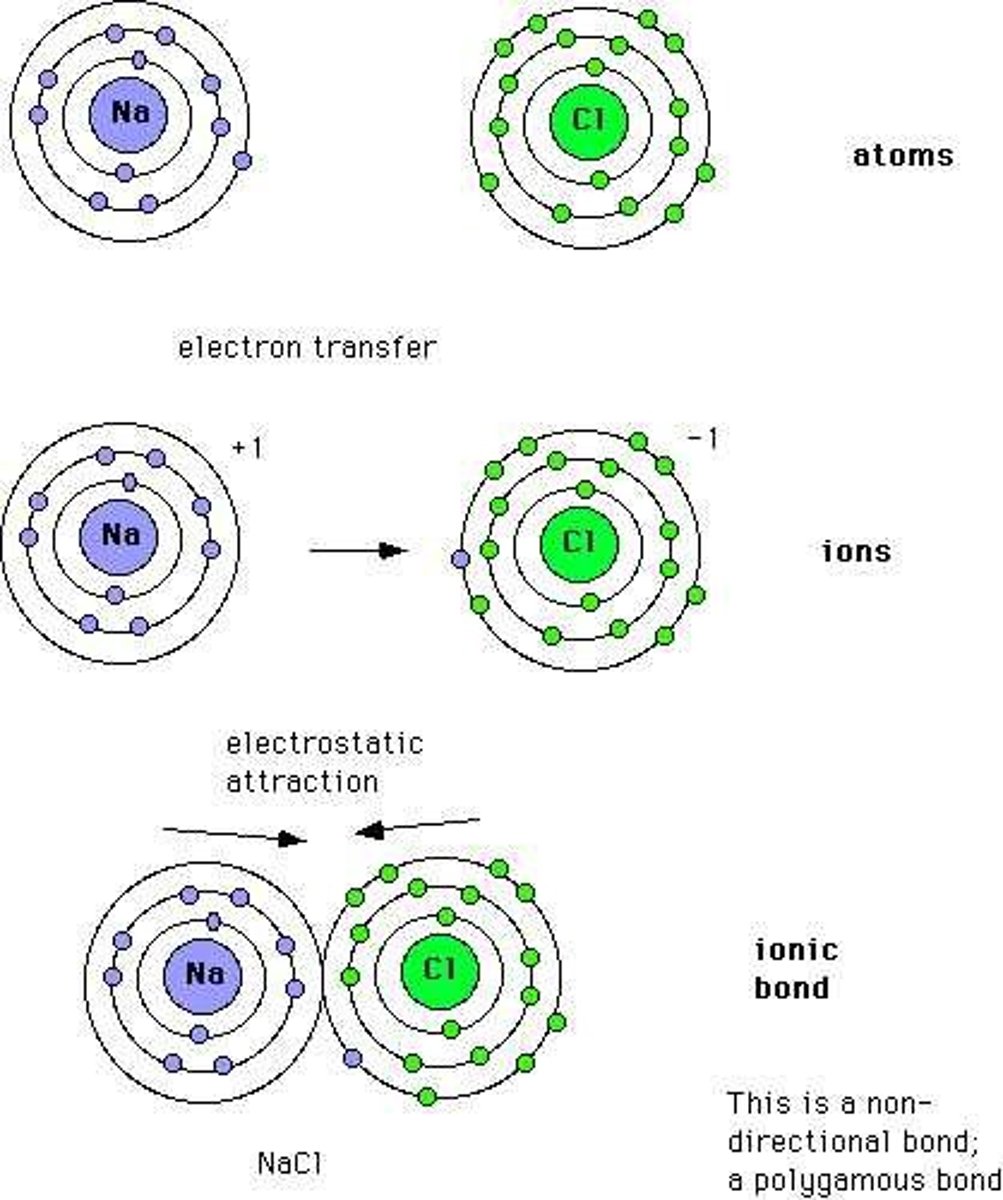
Calcium Hydroxide
Ca(OH)2
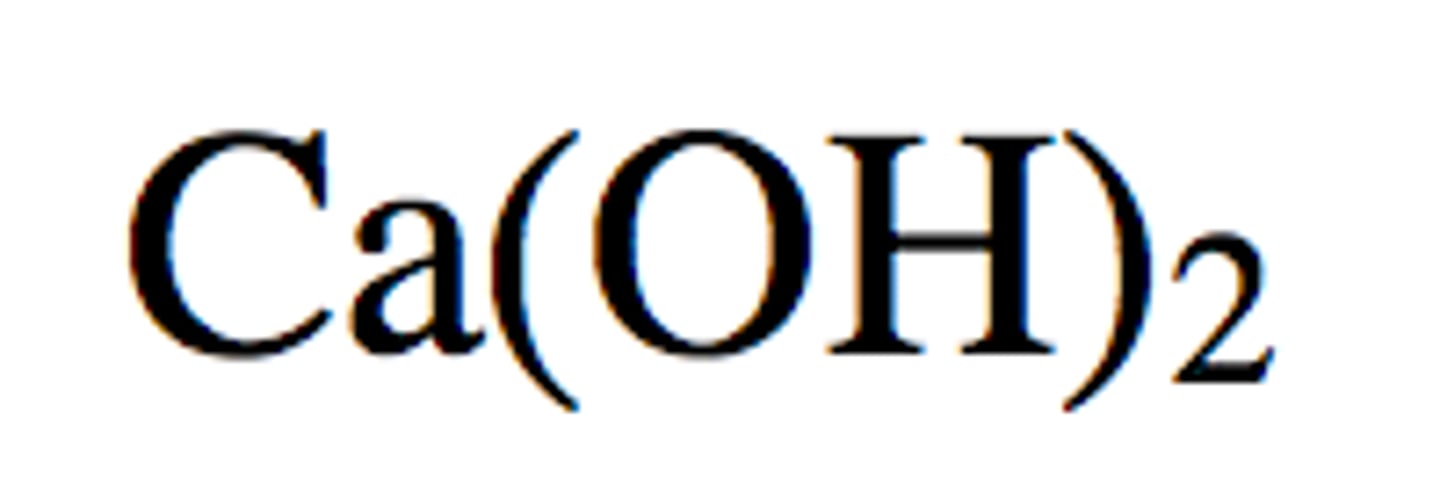
Sodium Hydroxide
NaOH
Calcium Carbonate
Ionic Compound, Formula is CaCO3

Lithium Hydroxide
Ionic Compound,Formula is LiOH

Cl2O
Dichlorine Monoxide
compound
contains two or more different elements chemically combines in a fixed proportion. can be broken down into simpler substance
Ionic Bonds
-bonding between a metal and non-metal or the bond between a positive ion and a negative ion forming a binary compound.
-end in the suffix "ide"
Covalent Bonds
• sharing of bonds between two non-metals
• end in "ide"
1
Mono
2
Di
3
Tri
4
Tetra
5
Penta
6
Hexa
7
Hepta
8
Octa
9
Nona