Enthalpy Change of Chemical Reactions: Physical Chemistry
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
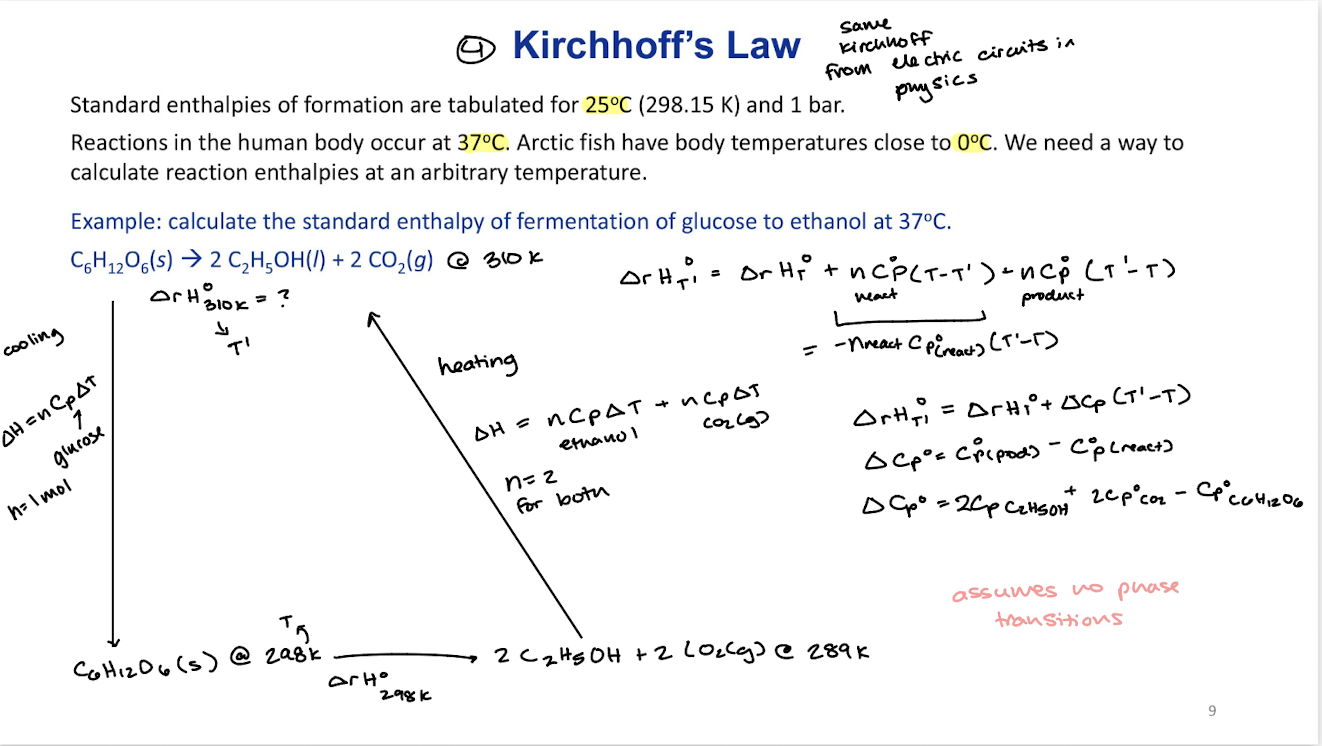
Standard enthalpy of formation (∆fH˚)
is the reaction enthalpy of formation of 1 mole the substance from elements in the reference state.
∆rH˚=∆fH˚(products)-∆fH˚(reactants)
Pay attention to state especially for water since there is a different ∆fH˚ of (l) and (g) H2O
if you change pressure ∆fH˚ changes
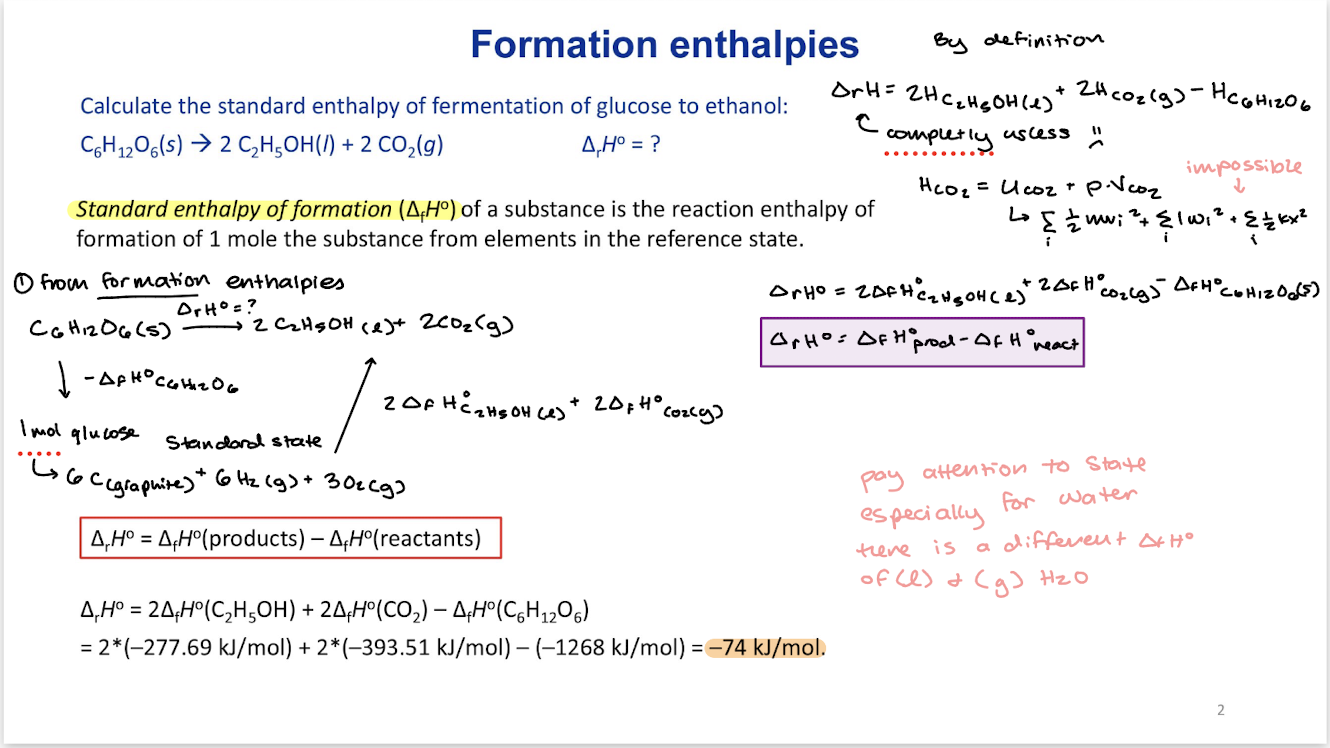
Bond enthalpies
Bond enthalpy is the reaction enthalpy of breaking a bond:
always > 0 because it takes energy to break a bond
Note 1: every atom is brought to gas phase to break every bond
Note 2: bond enthalpy somewhat depends on the molecule therefore, we use average bond enthalpies.
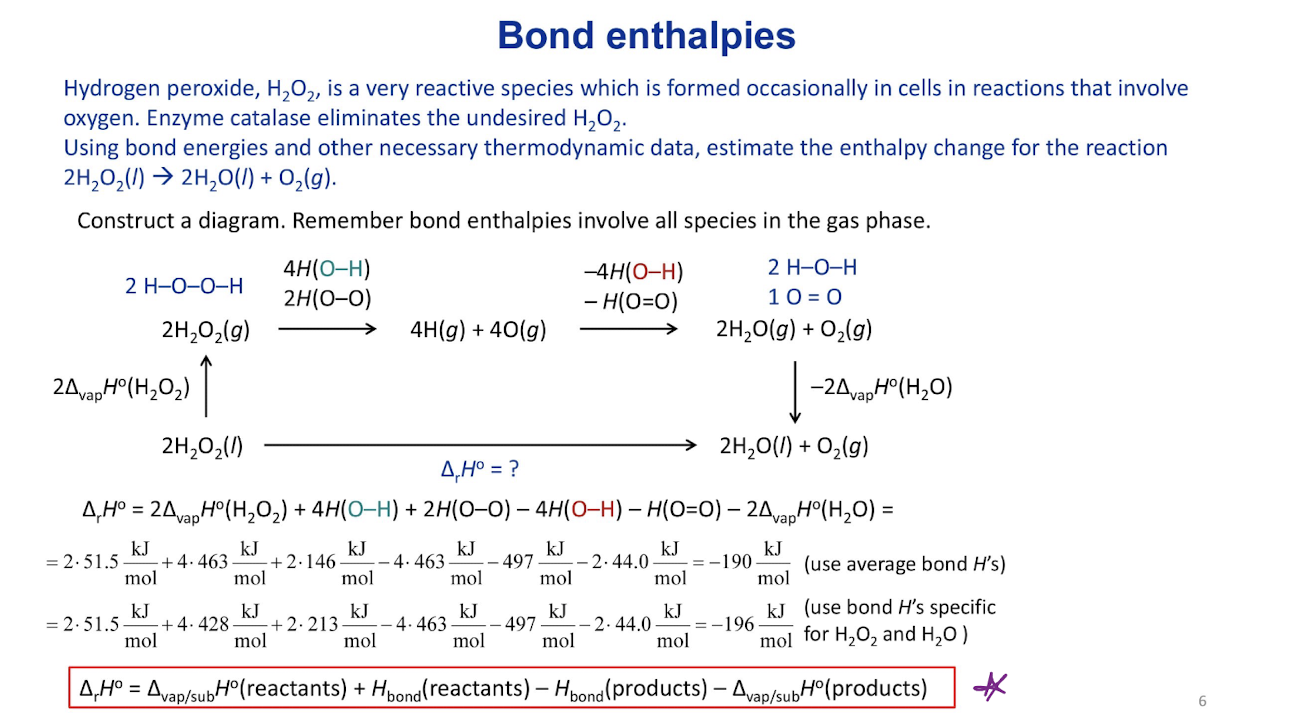
Combustion enthalpies
∆rH˚=∆combH˚(reactants)-∆combH˚(products)
Note 1: ∆cH˚ increases as the number of C atoms increases. Specific enthalpy and enthalpy density are better figures.
Note 2: Hydrocarbons have the most negative ∆cH˚, compared to other fuels with the same C atom count (compare CH4 and CH3OH).
Energy storage in organisms:
Fats (tristearin, C57H110O6): 38 kJ/g (9 kcal/g)
Carbohydrates (C6H12O6): 17 kJ/g (4 kcal/g)
Fats have a lower oxygen content per carbon and are more reduced than carbohydrates.
Fats offer the most compact way of energy storage.
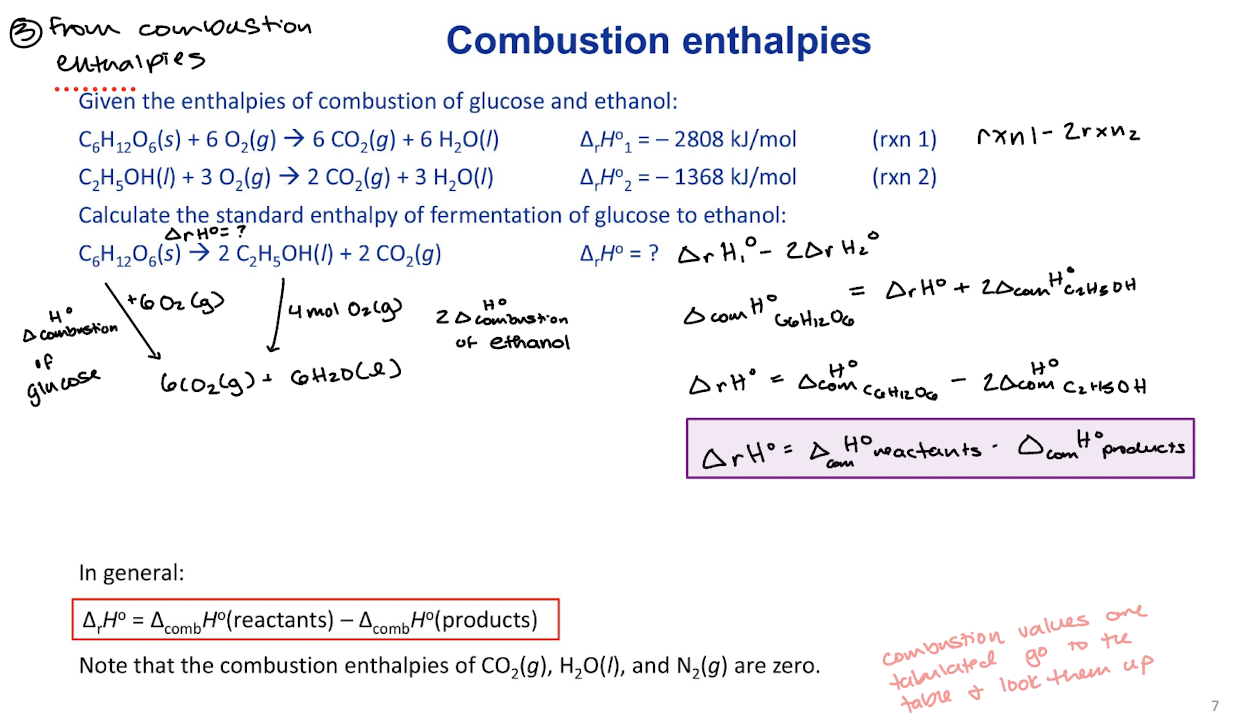
Kirchhoff’s Law