Module 5 Part I
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
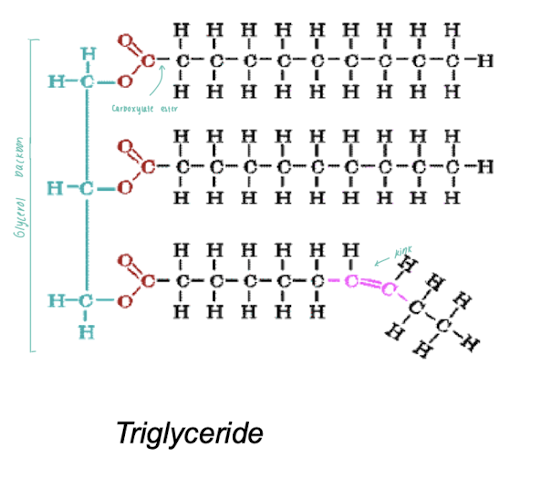
Identify the structural features of triglycerides.
Glycerol backbone
carboxylate ester
hydrocarbon side chain
different fatty acids
FAs can have different side chain lengths.
FAs can have saturated side chains.
FAs can have unsaturated side chains.
fuel
aid absorption of fat-soluble vitamins
Identify the structural features of phospholipids
glycerol backbone.
carboxylate ester
hydrocarbon side chain
phospho-ester
different fatty acids
FAs can have different side chain lengths.
FAs can have saturated side chains.
FAs can have unsaturated side chains.
Fuel
membrane
Cholesterol structure
synthesized in vivo.
animal source.
bile precursor
steroid precursor
membrane structure
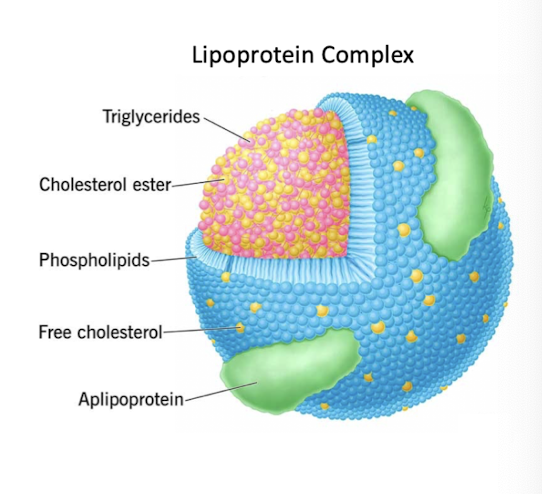
lipoprotein complexes
lipoprotein particles are complexes containing proteins and lipids
protein component consists of apoprotein – many members of this family
lipoprotein:
core of cholesterol esters and triglycerides
phospholipids, free cholesterol and apoproteins form outer layers.
What are the classes of lipoprotein complexes?
Classified according to density:
chylomicrons
very low-density lipoproteins
remnant particles, including intermediate-density lipoproteins
low-density lipoproteins
high-density lipoproteins
Used to transport of lipids to cells – cell-free lipids are poorly water saoluble
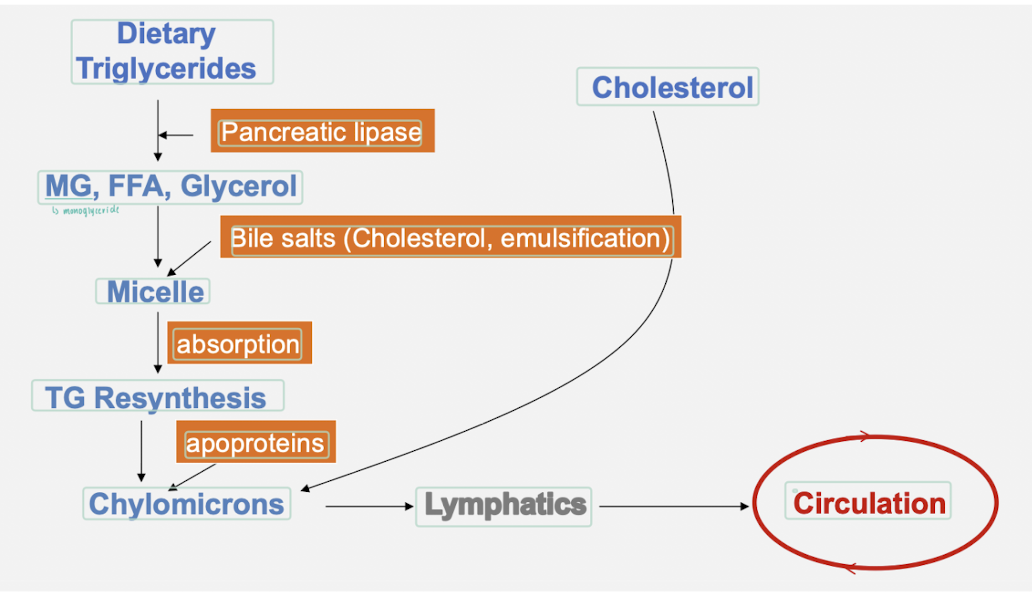
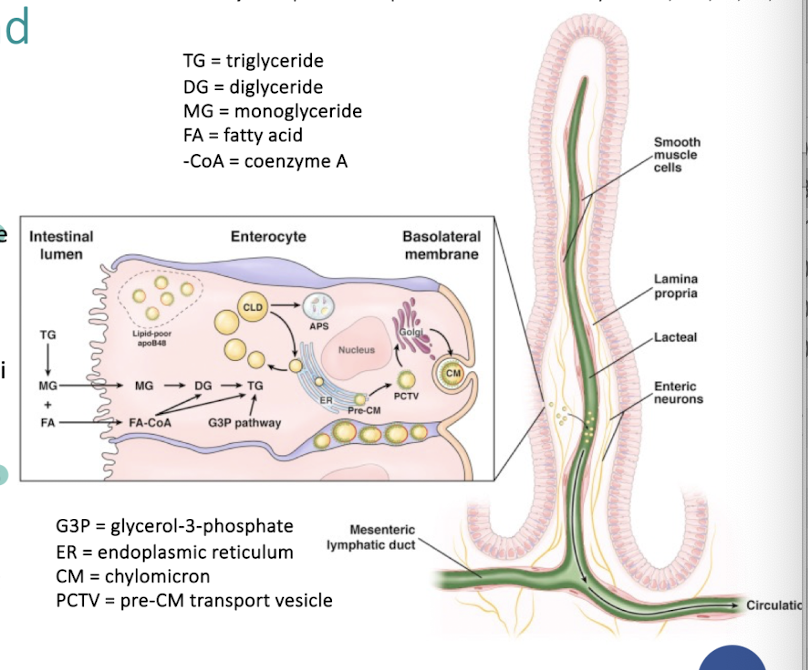
How are lipids absorbed from the gut?
How are chylomicrons made?
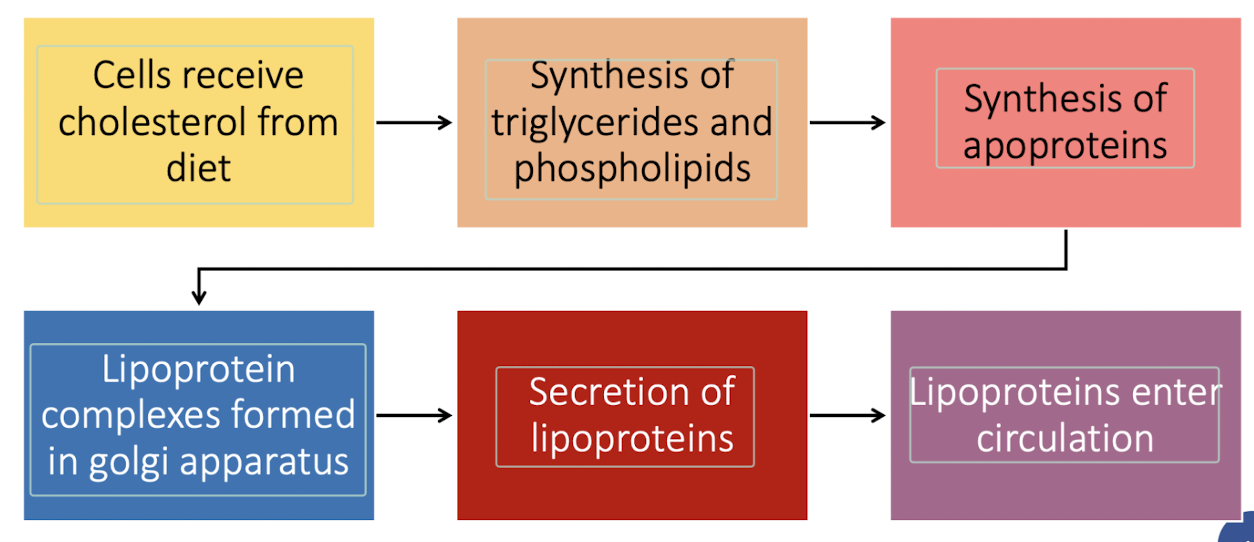
Explain the formation of chylomicrons synthesis and secretion
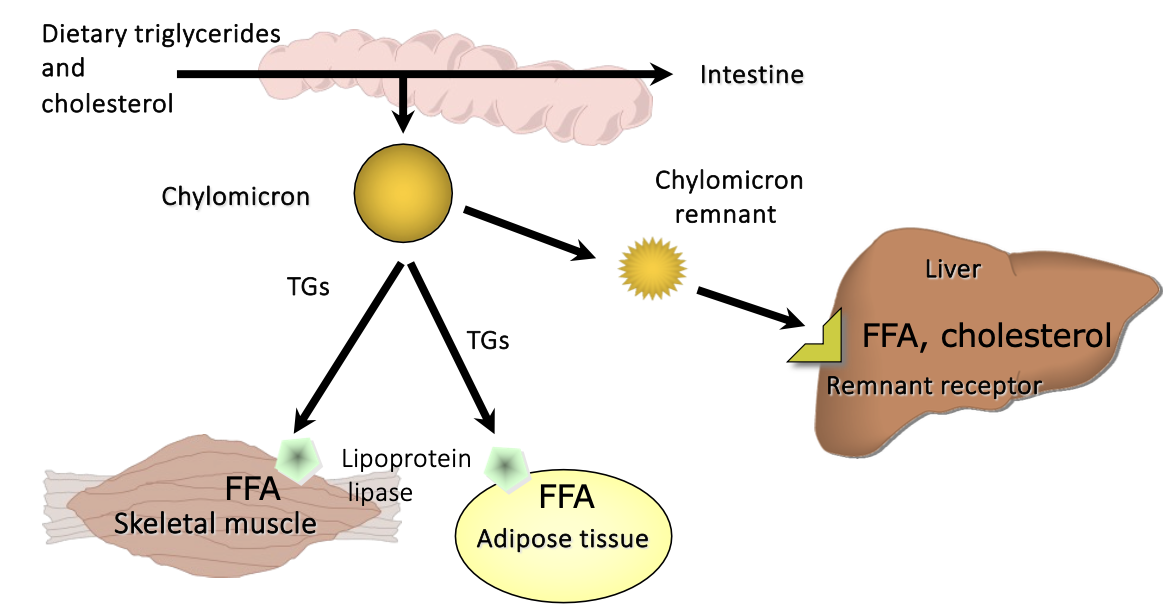
dietary triglycerides are digested to monoglycerides and fatty acids in the intestinal lumen.
monoglycerides, free fatty acids and cholesterol are absorbed at enterocyte brush border and cross apical membrane of the enterocyte.
lipid droplets formed in the ER membrane are packaged into pre-CMs and transported to the Golgi for processing.
mature CM particles exit basolateral membrane by exocytosis as secretory vesicles.
secreted CM particles move through lamina propria, enter lacteals, and are activity transported in lymphatic vessels of increasing size before being released into circulation
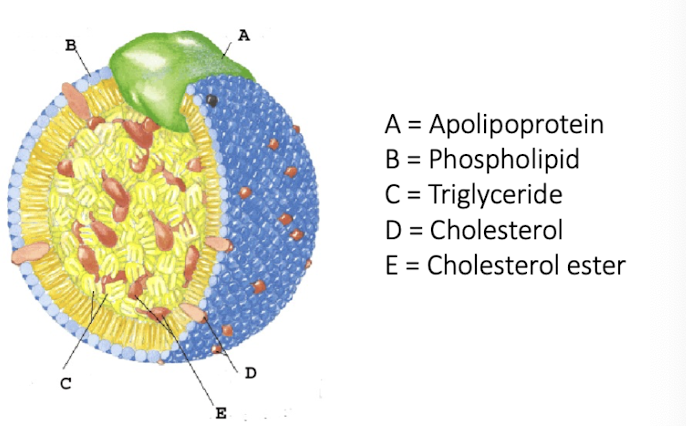
Chylomicrons - structure and composition
protein and lipid complex
50-200 nm in diameter
high proportion of triacylglycerol (85%)
transport dietary triglycerides from intestine to tissues
VLDL - structure and composition
protein and lipid complex
28-70 nm in diameter
approx. 50% triacylglycerols
transport TAGs synthesised in liver to adipose tissue and muscle
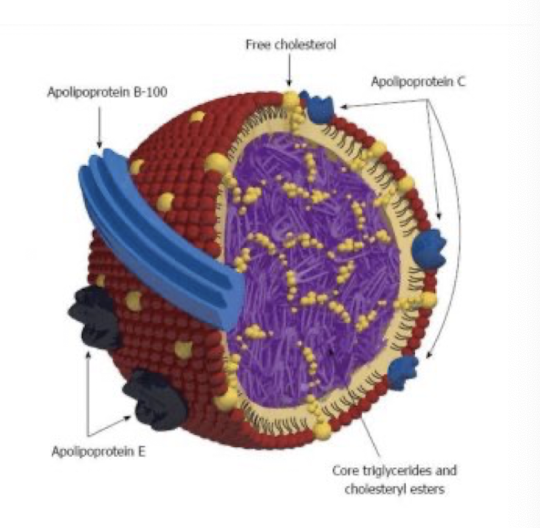
LDL - structure and composition
protein and lipid complex
20-25 nm in diameter
high in cholesterol (8%, 37% cholesterol esters).
protein 23%
formed from VLDL that has lost most of TAGs
carry cholesterol to non-hepatic tissues with apoB-100 receptors
HDL - structure and composition
8-11 nm in diameter protein and lipid complex
cholesterol 2%, cholesterol esters 15%, protein 55%
formed in liver as small protein-rich particles
contain enzymes to convert cholesterol from remnant chylomicrons and VLDL cholesterol to esters
collect cholesterol from peripheral tissues and return to liver
What are the three main functions of apolipoproteins
help solubilize cholesterol esters and triglycerides
regulate reactions of lipids with enzymes:lecithin: cholesterol acyltransferase, lipoprotein lipase, hepatic lipase
bind to cell surface receptors, determining the sites of uptake and rates of degradation of other lipoprotein constituents.
Apoprotein B
ApoB is the main class of non-exchangeable apoproteins triglyceride-rich lipoproteins.
ApoB-48 is produced in the intestine and is a component of chylomicrons.
ApoB-100 is produced in the liver and is a component of LDL, IDL, VLDL
ApoB-100 is the ligand for LDL receptor that permits LDL uptake.
ApoB-100 is encoded by the APOB100 gene
APOB100 gene mutations are associated with premature atherosclerosis
Apoprotein C-II
ApoC-II is an exchangeable apoprotein found on triglyceride-rich chylomicrons, VLDL, IDL, HDL
ApoC-II is a cofactor of lipoprotein lipase (LPL) that hydrolyses lipoprotein TG
ApoC-II is encoded by the APOC2 gene
APOC2 mutations are associated with hypertriglyceridemia
What is the fate of chylomicrons
Go to lacteals – lymphatic capillary in villi
Reach the bloodstream near the heart.
Deliver most of TG to adipose tissue and/or muscle
Degraded by different lipoprotein lipase in different tissues
Lipoprotein lipase is activated by apolipoprotein C-II made in liver and incorporated into chylomicrons
Lipoprotein remnants – cholesterol, apolipoproteins, phospholipids taken up by liver using apolipoprotein E
All dietary cholesterol is eventually transported to the liver
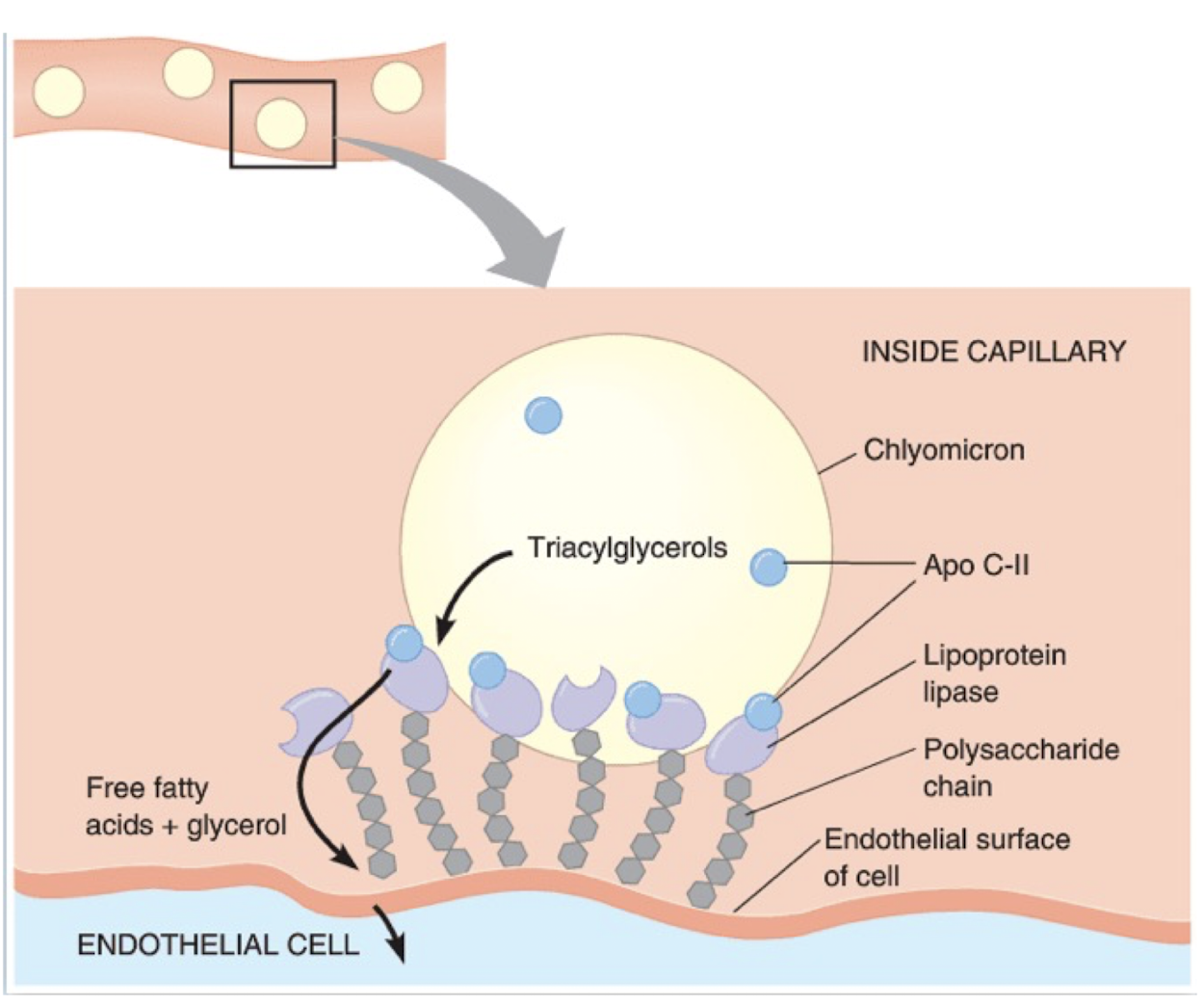
Functional role of lipoprotein lipases
Hydrolyse TG into two free fatty acids and one monoacylglycerol
Attached to luminal surface of endothelial cells in capillaries of adipose tissue
Ligand/bridging factor for receptor-mediated lipoprotein uptake
Hydrolyses extracellular triglycerides in lipoprotein complexes
Insulin activates lipoprotein lipase
Sites of cholesterol absorption and recovery
Plasma cholesterol is taken up by the liver, which converts cholesterol to bile salts by cholesterol-7-a-hydroxylase or packages it into LDL
Bile salts transported to gall bladder
Bile secreted into duodenum to aid lipid absorption, including cholesterol
Bile salts reabsorbed from the ileum.
Bile salts transported to liver and negatively regulates cholesterol-7-a-hydroxylase activity
In the liver
Before a meal:
Increase in cholesterol – exogenous and endogenous
Increase in fatty acids – exogenous from chylomicron remnants
Increase in fatty acids - endogenous formation from glucose
Between meals or if stressed:
Increase in fatty acids from adipose tissue
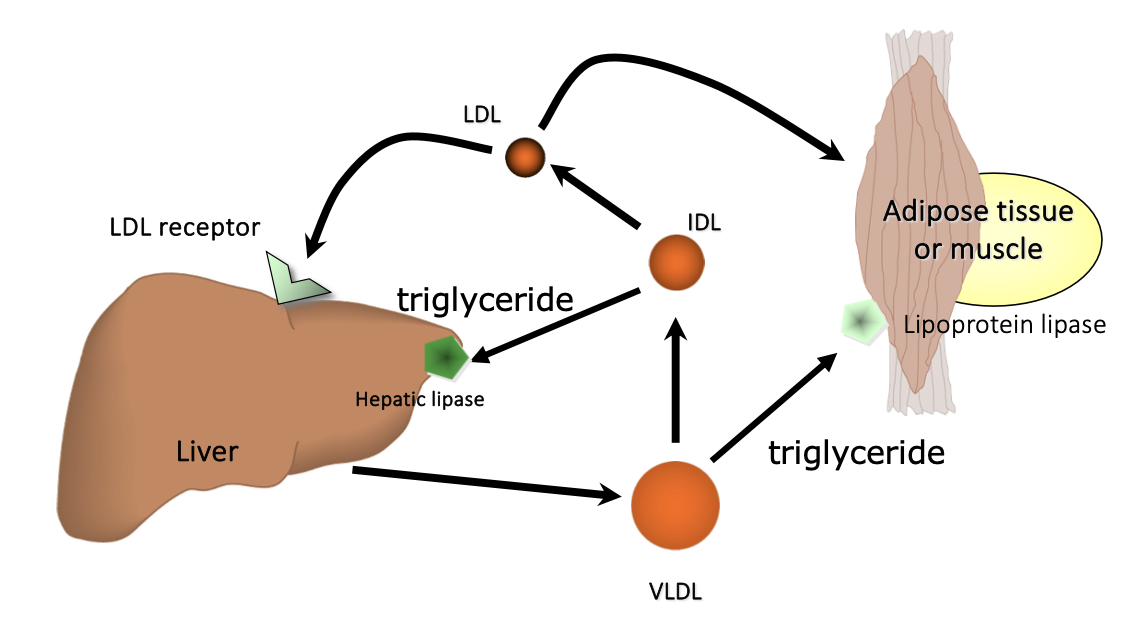
After a meal:
Increase in TG synthesis from fatty acids and glycerol
Increase in VLDL production
VLDLs are used to transport TG to peripheral tissues for storage or energy use
VLDL remnants form LDLs
Endogenous triglyceride synthesis and delivery
After a meal: LPL near adipose tissue is activated by insulin to store triglycerides (TG)
Between meals: skeletal muscle LPL is active and provides TG as a source of energy
When lipoprotein levels are lowest, heart muscle LPL has highest avidity for TGs and therefore heart continues to get energy
Explain the process of cholesterol metabolism
First process
Incorporated into VLDLs
delivered to cells in LDLs
esterification by lecithin: cholesterol acyl transferase
retrieved from cells in HDL’s
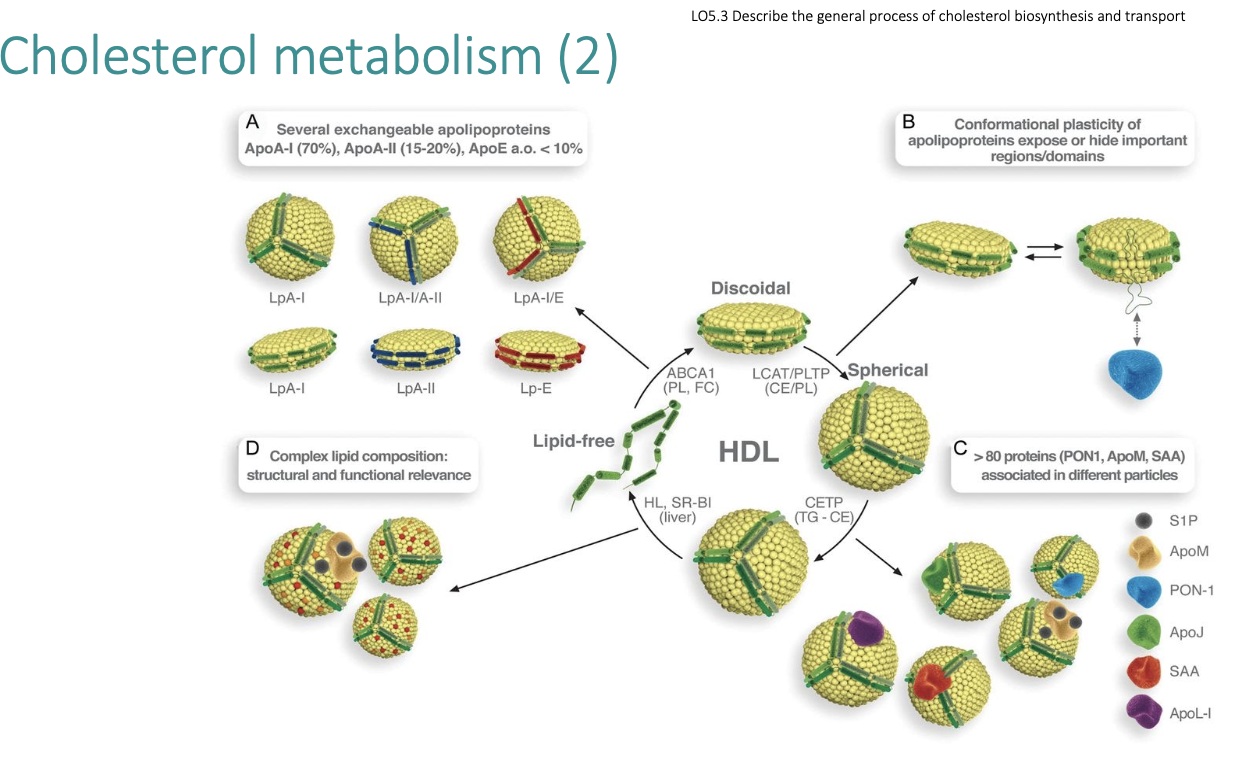
LDL metabolism
• 70% of cholesterol is in LDLs
• LDLs are transported to liver or peripheral tissue
• LDLs are taken up into cells intact
• Degradation of constituents occurs
• If no receptors LDLs can be taken up by scavenger cells (macrophages) leading to atherosclerosis
Delivery of Cholesterol to Peripheral Tissues
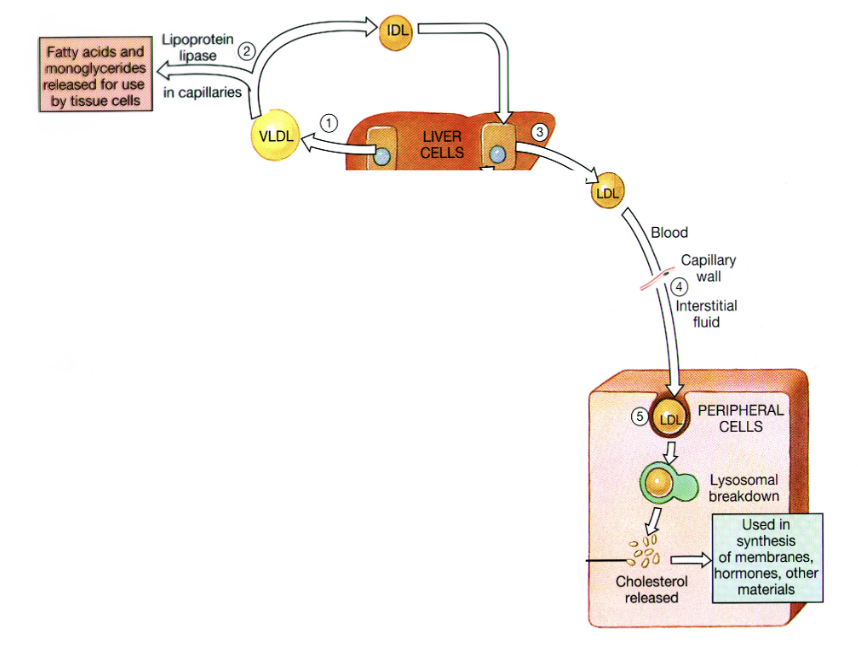
Uptake of Lipids from LDL
Complexes
LDL binds to ApoB-100
receptor-mediated endocytosis
LDL particles are degraded by lysosomes
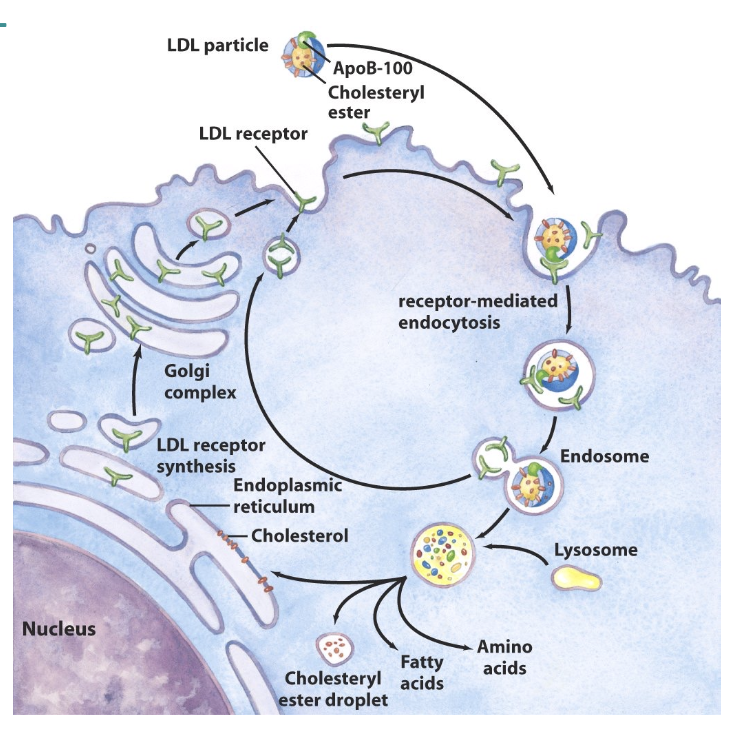
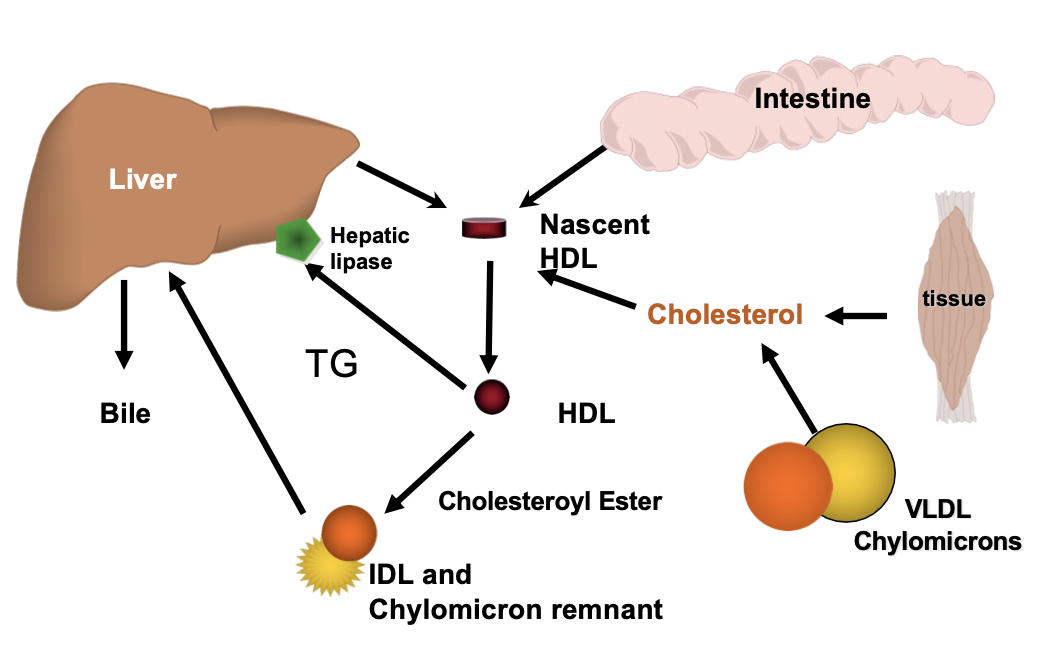
What is the fate of HDL complexes
• HDLs collect cholesterol
• LCAT is essential for the collection by HDL
• CETP assists in removal of cholesterol from peripheral sites
• HDLs are taken up into liver
• HDLs exchange cholesterol and apolipoproteins with other lipoproteins
Cholesterol esterification by Acyl-CoA:cholesterol acyltransferase (ACAT)
a) ACAT in the endoplasmic reticulum (ER) catalyses the covalent esterification of cholesterol by long-chain fatty acyl-CoAs to form cholesterol esters
b) Free cholesterol is found mainly in the plasma membrane & lysosomal membrane; transported by NPC1 and NPC2
lysosomal proteins to ER. Elevated cholesterol levels in ER inhibit cholesterol biosynthesis & decrease low-density
lipoprotein (LDL) receptors, increase cholesterol ester synthesis by ACAT enzymes. Cholesterol ester products of
NPC1/NPC2 the ACAT reaction are either stored in cytosolic droplets or secreted from cells as components of apoB-containing lipoproteins. In hepatocytes, cholesterol can also be converted to bile acids.
Recovery Pathway of Cholesterol from Peripheral Tissues
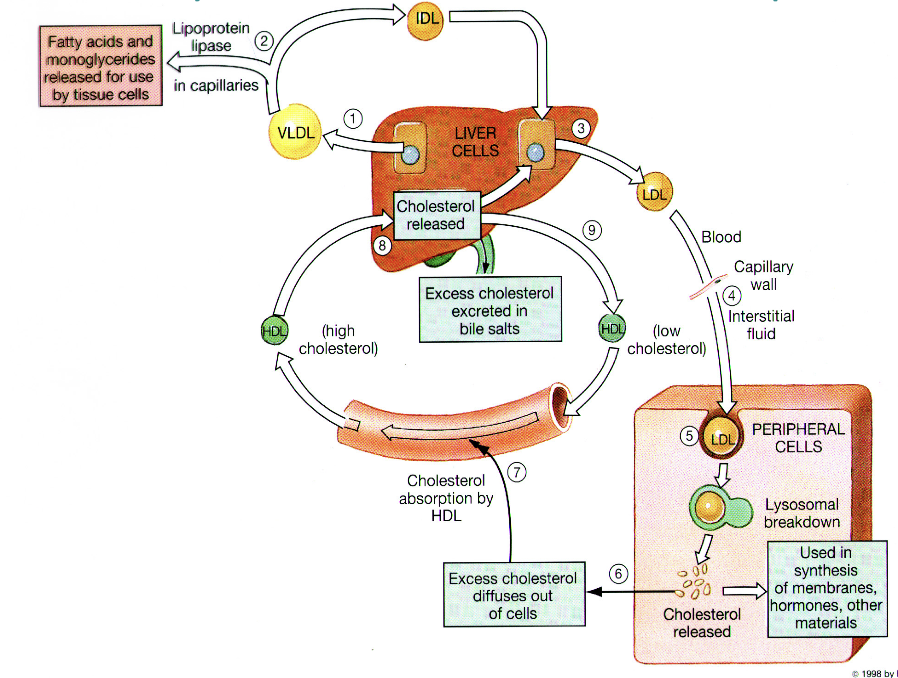
Exogenous pathway of lipid metabolism
Endogenous pathway of lipid metabolism
Reverse cholesterol transport
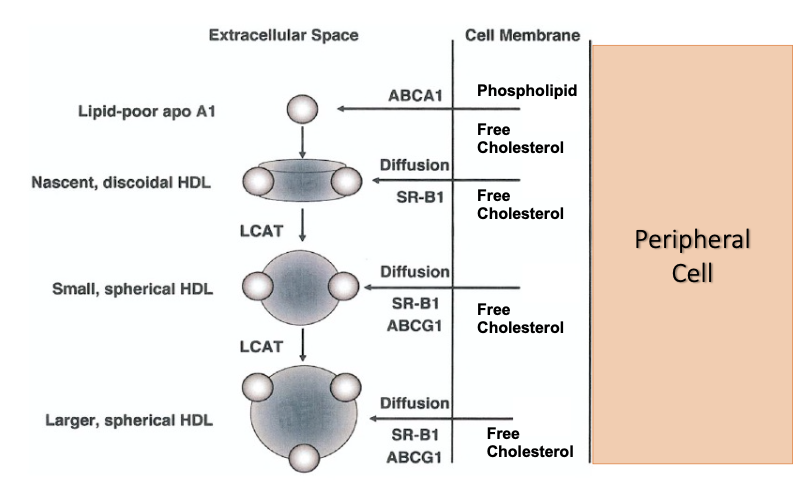
Lecithin:cholesterol acyltransferase (LCAT)
LCAT is a key enzyme in lipoprotein metabolism that enables the maturation of high-density lipoprotein (HDL) particles.
LCAT is activated by Apo A-1 and catalyses esterification of free cholesterol on the surface of HDL to form cholesteryl esters.
Cholesteryl esters partition into the lipoprotein core, resulting in the formation of mature spherical HDL particles.
LCAT and HDL maturation – Cholesterol esterification
Role of cholesteryl ester transfer protein
Cholesteryl ester transfer protein (CETP) promotes the transfer of cholesteryl esters from HDL to apoB-containing lipoproteins: VLDL, VLDL remnants, IDL, and LDL.
Deficiency of CETP is associated with increased HDL levels and decreased LDL levels, which is antiatherogenic