Chapter 9 HW Questions (Alkenes & Aromatics)
1/68
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
69 Terms
What is produced when benzene reacts with nitric acid in the presence of sulfuric acid?
nitrobenzene and water
what reagents are used to convert benzene to nitrobenzene
nitric acid and sulfuric acid
another name for toluene
methylbenzene
another name for anisole
methoxybenzene
hybridization of oxygen in anisole
sp2
another name for phenol
hydroxybenzene
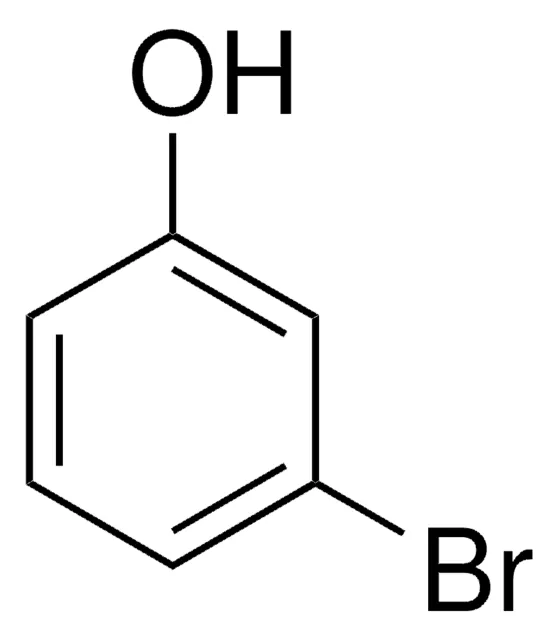
IUPAC name for this compound
3-bromophenol
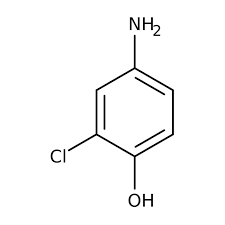
correct structure for 4-amino-2-chlorophenol
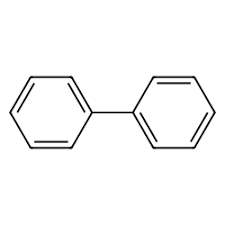
names of this comound
biphenyl
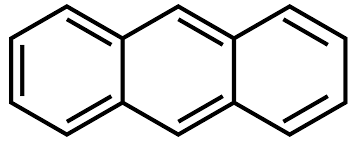
name of this compound
athracene
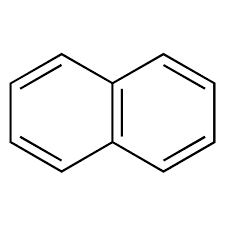
name of this compound
napthalene
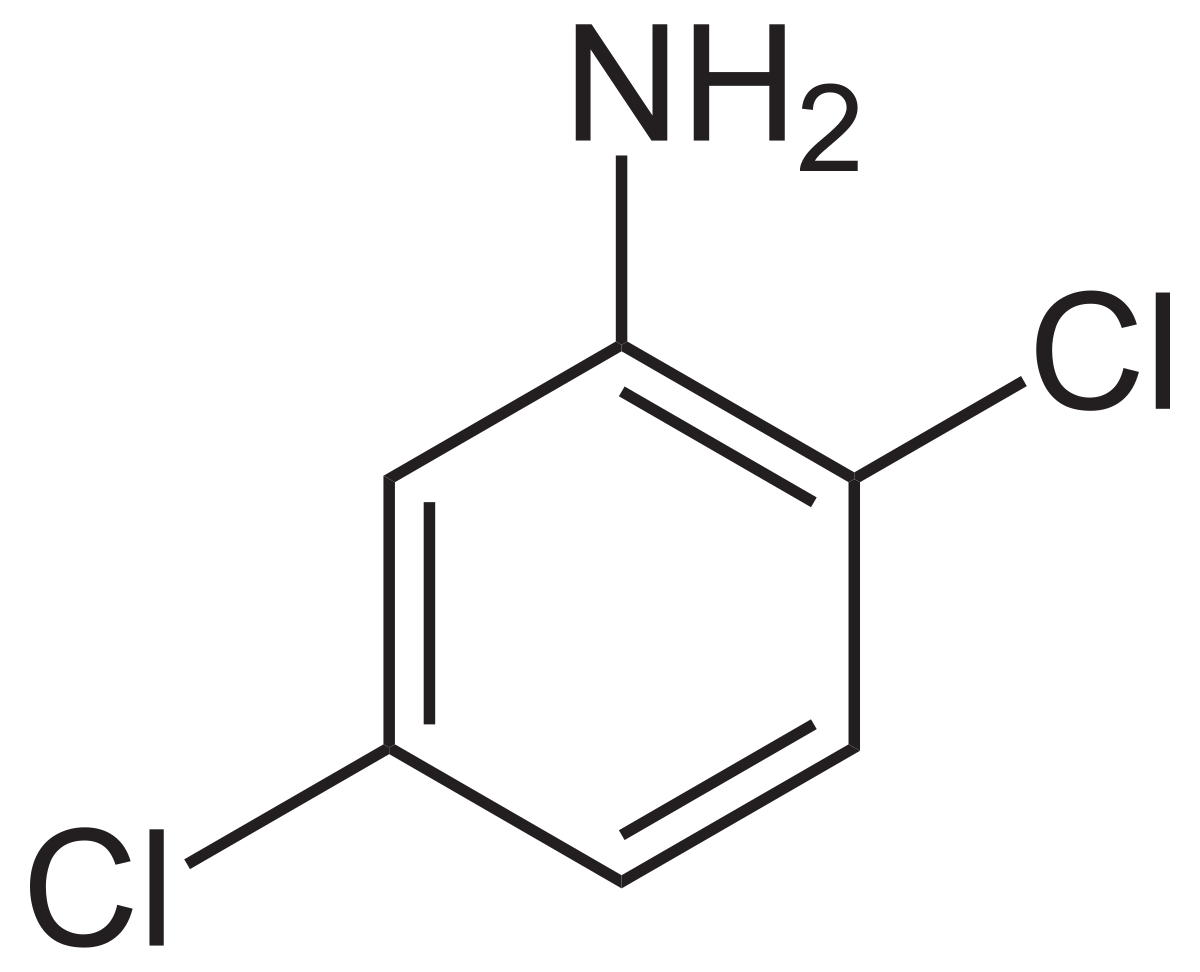
IUPAC name of this compound
2,5- dichloroaniline
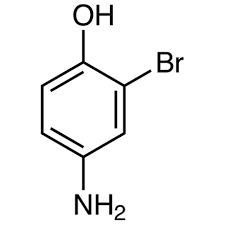
structure for 4-amino-2-bromophenol
what is cis trans isomerism associated with the presence of
double bonds and ring systems
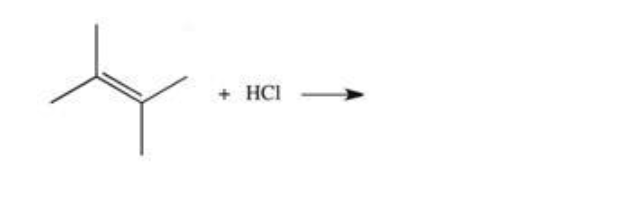
product of following reaction
it is an alkene reaction
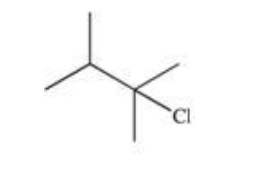
1-methylocyclohexene reacts with HBr to yield
1-bromo-1-methylcyclohexane
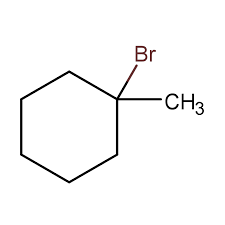
in an addition reaction to an alkene, the pi bond is
a nucleophile
addition reactions of alkenes are characterized by
the addition of two groups across a double bond
breaking of a pi bond
in the addition reaction of HI to 2-methyl-2-butene, what is the first step
formation of carbocation at carbon 3
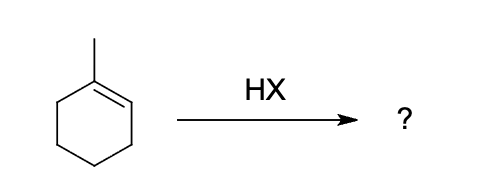
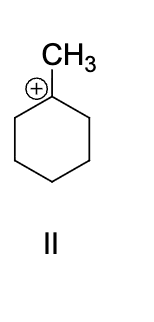
which structure depicts the most likely carbocation intermediate formed in the hydrohalogenation reaction shown
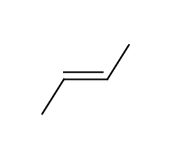
what is the expected product for the hydrohalogenation of the following alkene with HBr
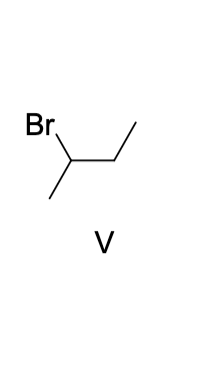
what is the expected Markovnikov addition product from the addition of HI to 2-methyl-2-butene
2-iodo-2-methylbutane
what is the expected anti-Markovnikov addition product from the addition of HI to 2-methyl-2-pentene
3-iodo-2-methylpentan
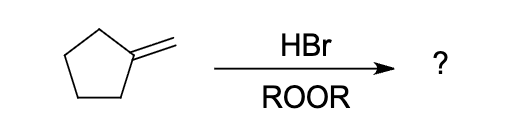
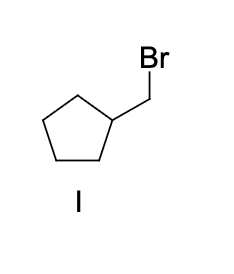
what is the expected major product of the following reaction
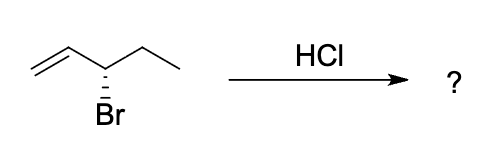
predict the expected major products of HCI addition to the alkene below
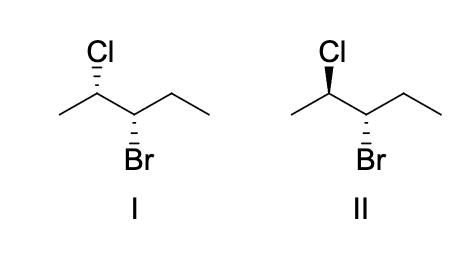
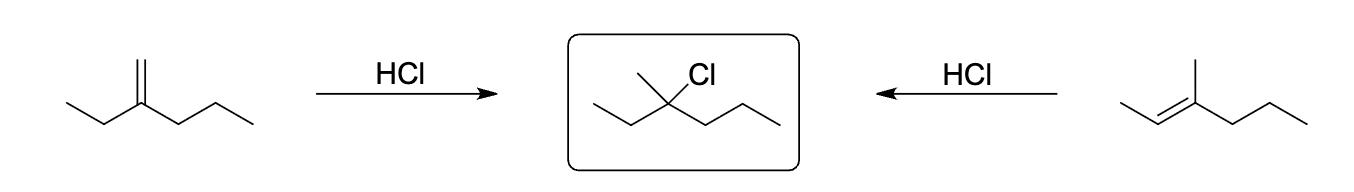
the product shown in the box can be produced by the treatment o each of the alkenes shown with HCI. Which reaction intermediate would explain this observation?
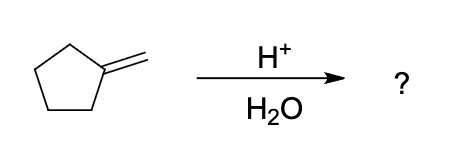
predict the expected major product of the following reaction
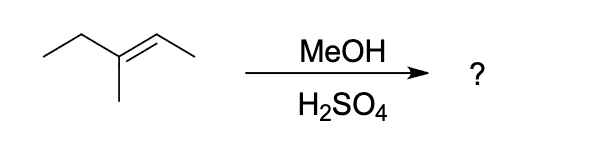
what is the expected major product of the following reaction
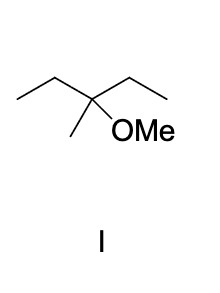
identify the compound that would react most slowly with a dilute aqueous solution of H2SO4
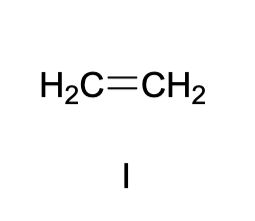
these three compounds can form a carbocation under aqueous acidic conditions, which will form the same carbocation
all three will form the same carbocation
what would the reaction of benzene with Br2/FeBr2 yield
bromobenzene
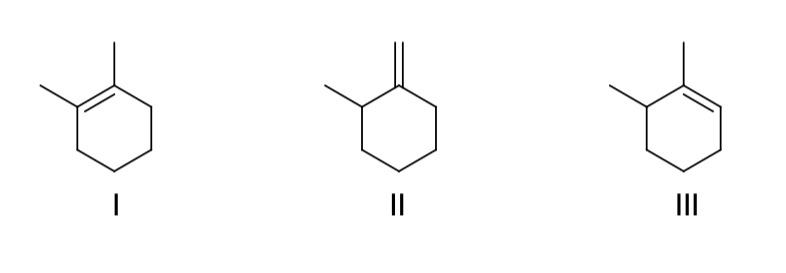
what are the major organic products generated from the reaction shown
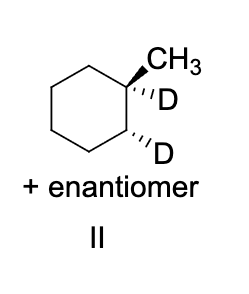
a bond angle of approximately 120 degrees is associated with the carbons in which functional group
alkenes
what is true of the 6 atoms of ethylene
all 6 atoms lie in the same plane
for which class of compounds does the lack of free rotation around carbon-carbon bonds result in the existence of cis-trans isomerism
alkenes
cis trans isomerism is associated with the presence of which of the following
double bonds and ring systems
what is true of cis trans isomerisms
they have the same structural connectivity
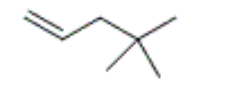
what is the correct IUPAC name of the following compound
4,4-dimethyl-1-pentene
what is the correct IUPAC name for the following compound
2,3-dimethyl-2-hexene
cis trans isomers exist for which of the following molecules, all of which have the molecular formula C3H5Br
1-bromopropene
which structure corresponds to cis-3-methyl-2-hexene
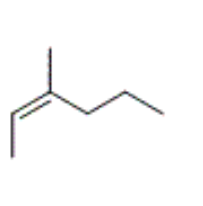
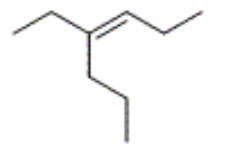
what is the correct IUPAC name for the following compound
cis-4-ethyl-3-heptene
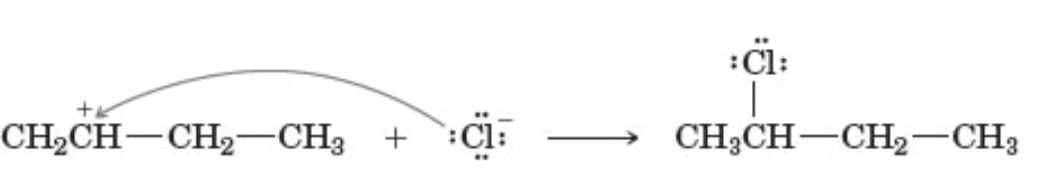
describe the reaction
involves a secondary carbocation
what is the product of the reaction of the substance given below with hydrogen and a Ni catalyst
butane
what reaction involves the addition of two equivalent groups to the two ends of a double bond
hydrogenation
which reactive involves the addition of two equivalent groups to the two ends of a double bond
bromination and hyrogenation
which of the curved arrows correctly describes the first step of the reaction of propene with hydrogen halide
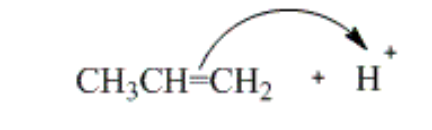
which curved arrow correctly describes the second step of the reaction of propene with hydrogen bromide
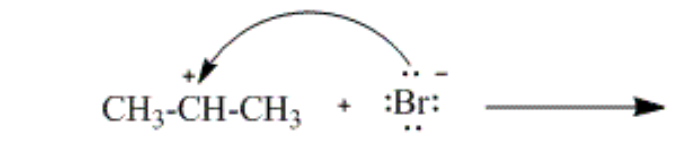
markovnikovs rule is not useful for predicting the outcome of the reaction of hydrogen chloride with which of the following alkenes
2-butene
markovnikovs rule is useful to predict the outcome of the reaction of hydrogen chloride with which of the following alkenes
1-butene
Markovnikovs rule is useful in predicting the outcome for which of the following addition reactions of unsymmetrical alkenes
hydration

which of the listed products will be formed in the following reaction
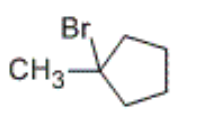

which of the following listed products will be formed in the following reaction
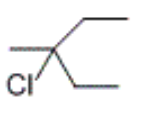
what product is obtained by the hydrobromination of 2-methyl-2-pentene
2-bromo-2-methylpentane
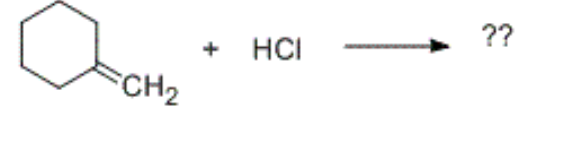
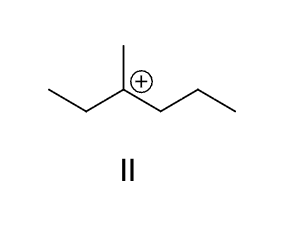
which of these carbocations is generated as an intermediate in the following reaction
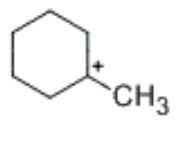
what is the charge of the organic intermediate formed during hydrogenation
+1
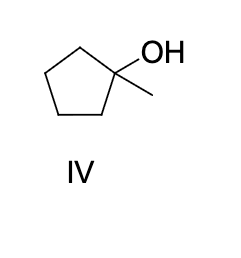
what type of alcohol is formed by the hydration of 1-butene
secondary
which alcohol is formed by the hydration of 2-methyl-2-pentene
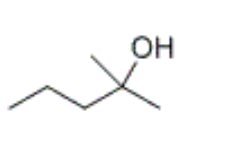
what types of intermediates are generated during the acid-catalyzed hydration of an alkene
oxonium ion
carbocation
product formed by the chloronation of 2-butene
2,3-dichlorobutane
product formed by the bromination of cyclohexene
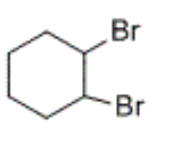
which of the following reactions requires a transition metal catalyst
hydrogenation
which of the following reactions is sulfuric acid used as a catalyst
hydration
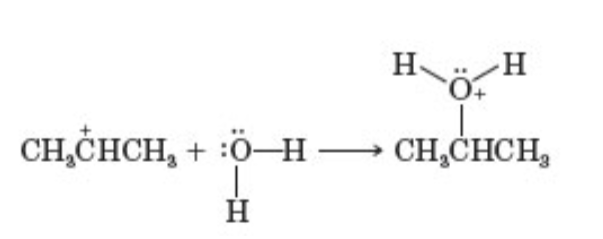
what pattern of organic reactions does this reaction belong to
reaction between an electrophile and nucleophile to form a new covalent bond
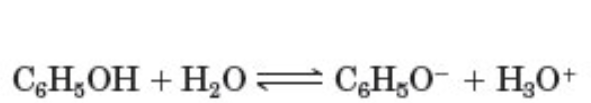
to which pattern does the following reaction belong
take a proton away
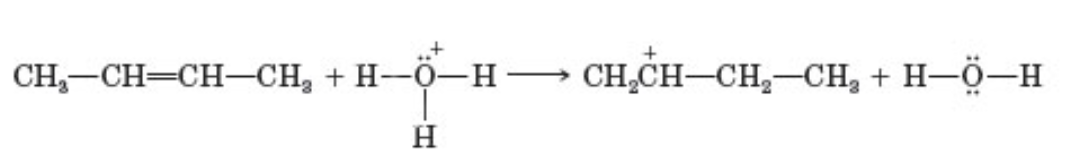
to which pattern does the following reaction belong
add a proton
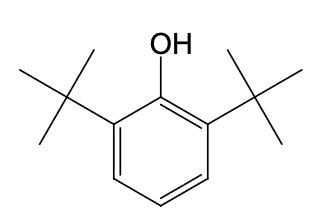
which of the following is expected to function as an antioxidant
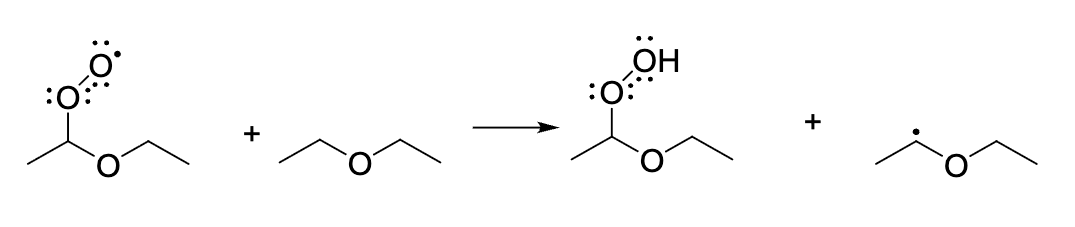
which term best describes the process shown
propagation
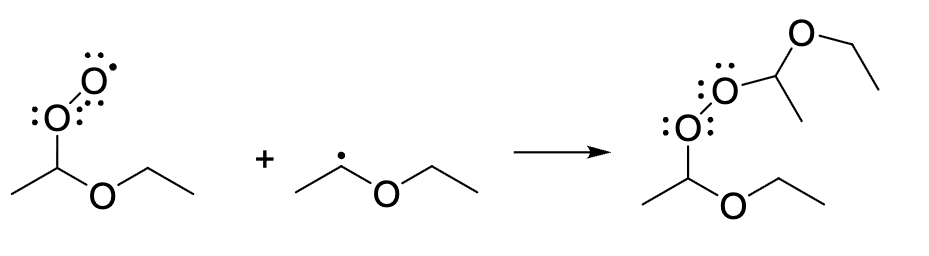
which term best describes the process shown
termination