Final Exam Review Slides
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
66 Terms
Which serological match between father and mother might result in HDFN?
Mother is negative for anti-K while father is positive for anti-K
Mother is Rh positive while father is Rh negative
Mother is positive for anti-Fya while father is positive for Fya antigen
Mother is negative for Lea antigen while father is positive for Leb antigen
Mother is positive for anti-Fya while father is positive for the Fya antigen
Why this one causes HDFN
Mother already has anti-Fya → IgG antibody, crosses the placenta.
Father is Fya-positive → fetus may inherit the Fya antigen.
If fetus is Fya-positive, maternal anti-Fya can bind fetal RBCs → hemolysis in utero or after birth → HDFN.
Which of the following features of an antibody increases the risk of HDFN
Compliment fixing
IgM
Antigens present on the placenta
Antigens fully developed at 16 weeks gestation
Antigens fully developed at 16 weeks gestation
Why
For HDFN to occur, the fetal antigen must be well-developed early in gestation so that if the mother has the corresponding IgG antibody, it can bind and destroy fetal RBCs in utero.
Many clinically significant RBC antigens (like D, c, K) are well developed by 16 weeks — meaning damage can begin before birth.
Which of the following is the most common cause of HDFN?
Anti-A,B
Anti-D
Anti-K
Anti-C
Anti-A,B
Why
ABO HDFN (most often from maternal group O with IgG anti-A,B) is more common than Rh-mediated HDFN because:
ABO incompatibility between mother and fetus happens frequently.
Anti-A,B in group O mothers can cross the placenta (IgG form).
However: it’s usually mild compared to anti-D HDFN — fewer fetal RBCs express ABO antigens at birth, and they’re not as well developed in utero.
Why not the others
Anti-D – Most severe form of HDFN, but less common today because of RhIg prophylaxis.
Anti-K – Important cause of severe HDFN, but much less common in general population.
Anti-C – Can cause HDFN, usually less severe than anti-D or anti-K, and not as frequent.
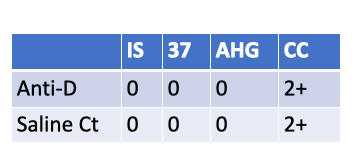
What is the Rh type of a newborn with the following serological results?
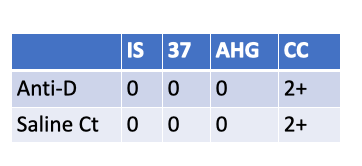
Rh-negative newborn
These results show:
Anti-D: No agglutination at Immediate Spin, 37 °C, or AHG phase (all 0).
Saline control: Also all negative.
Check cells: 2+ → confirms AHG phase was valid (reagent working).
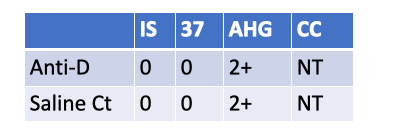
What follow up testing should be performed on a newborn with the following serological results?
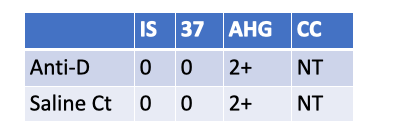
Eluate
DAT
Maternal Antibody screen
Wash the cells a repeat the WeakD test
DAT.
Reason: Weak D is 2+ at AHG, but the saline control is also 2+ at AHG → the weak D test is invalid (suggests the newborn’s RBCs are IgG-coated, so AHG will agglutinate with or without anti-D). The correct follow-up is a DAT to confirm in-vivo coating.
If DAT is positive, next step is an eluate to identify the antibody (often maternal anti-D/other IgG). Re-washing and repeating weak D won’t fix a truly IgG-coated cell situation.
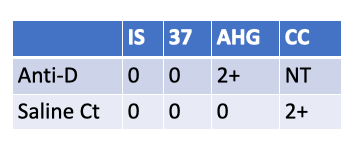
Which other test is invalidated by the following serological results on a newborn screen?
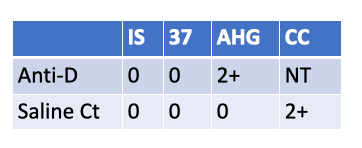
KLB
FMH
Rhogam
Maternal Rh type
FMH (rosette) screen.
Because the newborn is Weak D positive (Anti-D 2+ at AHG with a valid negative saline control), the rosette test on the D-negative mother can be falsely negative—some anti-D reagents used in the rosette assay don’t reliably detect weak D on fetal cells. In this situation, skip/override the FMH screen and go directly to Kleihauer-Betke (or flow cytometry) to quantify FMH for RhIg dosing.
Under what circumstances would the fetal screen be skipped in favor of a KLB?
Trauma
STAT Rhogam order
When the mother is Rh positive
When the mother’s antibody is not anti-D
Trauma
Which of the following cells would you choose as a titer cell when evaluating HDF caused by anti-Jka?
R0r,K-/k,Fy(a-b+)Jk(a-b+)S+s+M-N+
R1R1,K+/k,Fy(a+b-)Jk(a-b+)S+s+M+N+
rr,K-/k,Fy(a+b-)Jk(a+b-)S-s+M-N+
R2r,K+/k,Fy(a-b+)Jk(a+b+)S+s-M+N-
rr, K-/k-, Fy(a+b-), Jk(a+b-), S-s+, M-N+
✅ Jk(a+b-) → Expresses Jkᵃ antigen (required for anti-Jkᵃ detection).
✅ K-, Fy(b-) → No interference from anti-K or anti-Fyᵃ.
✅ Rh-negative (rr) → Safe for testing Rh-negative mothers.
For an anti-Jkᵃ titer, pick a Jkᵃ-positive cell that’s homozygous (Jk(a+b-)) to maximize reactivity via dosage.
The other options are either Jkᵃ-negative [Jk(a-b+)] or heterozygous [Jk(a+b+)] and are not preferred for titration.
Which HDFN test does the Flow Cytometry test replace?
KLB
Fetal Screen
Cordblood Workup
Antibody Titer
KLB (Kleihauer-Betke test)
Why
Flow cytometry using fluorescent anti-HbF (fetal hemoglobin) or anti-D is a more sensitive and precise way to quantify fetal cells in maternal circulation.
It replaces the Kleihauer-Betke acid elution smear method for quantitative FMH testing, especially when calculating RhIg dose.
Some labs still run KB, but flow cytometry is preferred where available because it’s less subjective and more accurate.
Which patient is disqualified from receiving Rhogam?
Mary who is O negative Weak D positive with a negative antibody screen in her 2nd Trimester
Sonya who is A negative with a positive antibody screen for anti-Lea in her 1st trimester
June who is B negative with a negative antibody screen is newly delivered of an Rh negative set of twins
Candace is O negative with a positive antibody screen for anti-E is newly delivered of an O positive baby
Mary — O negative, Weak D positive.
Why: A Weak D–positive mother is managed as Rh(D) positive (on exams unless genotyped otherwise), so she’s not a candidate for RhIG.
Sonya (A−, anti-Leᵃ): Still Rh− with no anti-D → RhIG indicated.
June (B−, Rh− twins): Postpartum RhIG not indicated this delivery (babies are Rh−), but she’s not “disqualified” in general.
Candace (O−, anti-E, Rh+ baby): RhIG indicated; anti-E doesn’t affect RhIG use.
The MLS has found 34 fetal cells/1000 on Sunny’s KLB slide. How many doses of Rhogam does she require?
1
3
5
7
7
formula is the %of fetal cells (34/1000) x 5,000 / 30
0.034 × 5000 / 30 =5.667→ round 6 +1 for added safety margin = 7 doses Rhogam
Hydrops fetalis is caused by:
Unconjugated bilirubin deposits
Anemia
Maternal Antibody Titer
Maternal Kidney compensation
Anemia
Why
In severe HDFN, maternal IgG antibodies destroy large numbers of fetal RBCs → severe anemia.
This anemia triggers high-output cardiac failure, generalized edema, and effusions → hydrops fetalis.
Why not the others
Unconjugated bilirubin deposits ❌ — Cause jaundice/kernicterus after birth, but not hydrops in utero (placenta clears bilirubin before birth).
Maternal antibody titer ❌ — High titer is a risk indicator, not a direct cause of hydrops.
Maternal kidney compensation ❌ — Not related to the pathophysiology of hydrops fetalis.
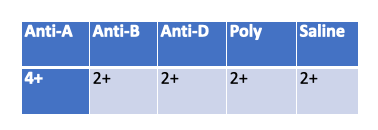
A cordblood workup is performed on Baby girl Springfield. According to her results does she have HDN?
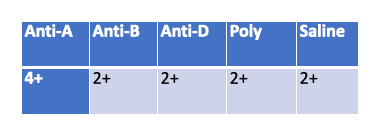
Yes
No
Invalid
Invalid
Why
In a cord blood workup, the saline control is meant to detect spontaneous agglutination (false positives due to cold agglutinins, rouleaux, or strong autoantibodies).
A positive saline control (2+) means the reactions with Anti-A, Anti-B, Anti-D, and Poly could be false — the results cannot be interpreted
Next step
Wash the baby’s RBCs thoroughly to remove excess protein or maternal antibody coating causing nonspecific agglutination.
Repeat the testing to obtain valid ABO, Rh, and DAT results.
Anti-E is detected in the serum of a woman in the first trimester of pregnancy. The first titer for anti-E is 32. Two weeks later, the antibody titer is 64 and then 128 after another 2 weeks. Clinically there are beginning to be signs of fetal distress. What can be done?
Induce labor for early delivery
Perform plasmapheresis
Administer Rhogam
Intrauterine exchange transfusion
Intrauterine exchange transfusion
Why
The woman has rising anti-E titers (32 → 64 → 128) and signs of fetal distress, which indicates significant HDFN from anti-E.
At this stage, Rhogam is useless (it’s only for prevention of anti-D, not treatment).
Plasmapheresis is sometimes used early in pregnancy to lower maternal antibody levels, but here the fetus is already in distress — urgent direct treatment is needed.
Early induction might be considered if the fetus is near term, but in this case, an intrauterine transfusion (IUT) is the standard intervention to correct anemia and allow the pregnancy to continue until safer delivery.
What is IUT: A procedure in which donor red blood cells are transfused directly to the fetus while still in the womb.
Purpose in HDFN
Used when maternal antibodies (like anti-D, anti-K, anti-E) are destroying fetal RBCs, causing severe anemia.
Corrects anemia, improves oxygen delivery, and prevents hydrops fetalis or fetal death.
Allows the pregnancy to continue until the fetus is mature enough for safe delivery.
What testing is performed on maternal plasma in preparation for a neonate exchange transfusion?
ABO Rh only
Crossmatch and Antibody screen
Type and Screen
Type and DAT
Crossmatch and Antibody Screen
Why
In an exchange transfusion for a neonate, the blood used must be compatible with both the neonate and the mother.
Since maternal antibodies are the cause of HDFN, we test maternal plasma to:
Antibody screen – Detect any clinically significant antibodies.
Crossmatch – Ensure the donor RBCs are antigen-negative for those antibodies and compatible with maternal plasma.
Why not the others
ABO Rh only ❌ – Not enough; misses unexpected antibodies.
Type and Screen ❌ – The “screen” is done, but you also need the actual crossmatch with donor units.
Type and DAT ❌ – DAT is for detecting antibody-coated RBCs, not selecting compatible donor blood.
What type of blood product would you provide for a newborn suffering from alloimmune thrombocytopenia?
HLA matched platelets
CMV neg, Sickle Cell neg fresh RBCs
Crossmatched platelets
Irradiated platelets
Crossmatched platelets…but double check with Rachelle
Why
Neonatal alloimmune thrombocytopenia (NAIT) occurs when maternal antibodies target paternal platelet antigens inherited by the fetus.
The safest product is antigen-negative platelets — ideally from the mother (washed/irradiated) or crossmatch-compatible donor platelets.
Crossmatching ensures the platelets lack the antigen being targeted and will survive in circulation.
Why not the others
HLA matched platelets ❌ – Used for platelet refractoriness due to HLA alloimmunization, not NAIT.
CMV neg, Sickle Cell neg fresh RBCs ❌ – RBCs are irrelevant here; the problem is platelets.
Irradiated platelets ❌ – Irradiation prevents graft-vs-host disease but does not address the antigen incompatibility causing NAIT. (Platelets for NAIT are often irradiated in addition to being crossmatched, but “crossmatch” is the key selection factor.)
Which of the following is not a symptom of acute hemolytic transfusion reaction?
Back pain
Shortness of breath
Fever
Watery stool
Watery stool
Why
Acute hemolytic transfusion reaction (AHTR) is most often due to ABO incompatibility, causing intravascular hemolysis.
Classic symptoms appear during or shortly after transfusion and include:
Fever/chills
Back/flank pain
Hypotension
Shortness of breath/dyspnea
Hemoglobinuria
Sometimes chest pain, anxiety, or nausea/vomiting
Watery stool is not a hallmark sign — GI symptoms are nonspecific and not a defining feature of AHTR.
Delayed transfusion reactions tend to be associated with antibodies that:
Cause Extravascular hemolysis
Cause Intravascular hemolysis
Are IgM
Appear within 24 hours
Cause extravascular hemolysis
Why
Delayed hemolytic transfusion reactions (DHTRs) usually occur days to weeks after transfusion.
They are most often caused by IgG antibodies (e.g., Kidd, Duffy, Kell, Rh) that opsonize donor RBCs, leading to extravascular destruction in the spleen/liver.
Commonly due to anamnestic response — antibody was previously undetectable, then reappears after re-exposure.
Why not the others
Intravascular hemolysis ❌ – More common in acute hemolytic reactions (e.g., ABO incompatibility).
IgM ❌ – Usually cause immediate intravascular hemolysis, not delayed.
Appear within 24 hours ❌ – DHTRs occur >24 hours post-transfusion (often 3–14 days).
Febrile transfusion reactions can be prevented by which of the following actions?
Transfusing irradiated products
Pre treatment with Benadryl
Pre treatment with Tylenol
Transfusing HLA matched products
Transfusing HLA-matched products
Why?
Febrile non-hemolytic transfusion reactions (FNHTR) are often caused by cytokine release from donor leukocytes or recipient antibodies reacting against donor HLA antigens.
HLA-matched products reduce the risk of HLA-mediated immune reactions, preventing FNHTRs in patients with a history of reactions (e.g., multiparous women or multiply transfused patients).
Why Not the Other Options?
Transfusing irradiated products
Prevents TA-GVHD (Transfusion-Associated Graft-vs-Host Disease) but does not prevent FNHTRs.
Pretreatment with Benadryl (diphenhydramine)
Used for allergic reactions (e.g., urticaria), not febrile reactions (which are not histamine-mediated).
Pretreatment with Tylenol (acetaminophen)
May reduce fever symptoms but does not prevent the reaction itself.
Urticarial reactions are usually caused by what?
IgM antibodies in red cells
IgA antibodies in plasma products
Leukocytes in non-irradiated products
Protein allergens in platelets
Protein allergens in platelets
Why
Urticarial (allergic) transfusion reactions are usually mild and caused by the recipient’s immune response to plasma proteins in the donor product.
Platelets contain a large volume of plasma, so they’re more likely than RBCs to trigger allergic reactions — especially urticaria/hives.
These reactions are typically mediated by IgE, not IgM or IgA, and present with itching, hives, flushing (without fever or hemolysis).
Why not the others
IgM antibodies in red cells ❌ – Associated with acute hemolytic reactions (e.g., ABO incompatibility).
IgA antibodies in plasma products ❌ – Cause anaphylactic reactions in IgA-deficient patients, not mild urticaria.
Leukocytes in non-irradiated products ❌ – Cause TA-GVHD risk, not urticarial reactions.
Patients most at risk of anaphylactic reactions to blood component transfusion are
Allergic to IgA antibodies
Allergic to shellfish
Receiving blood products from relatives
Cardiovascularly limited
Allergic to IgA antibodies
Why
Severe anaphylactic transfusion reactions most often occur in IgA-deficient patients who have formed anti-IgA antibodies.
When transfused with plasma-containing blood products from a normal donor (which contain IgA), the anti-IgA triggers a rapid, severe allergic response — hypotension, bronchospasm, shock.
These patients require washed cellular components or IgA-deficient plasma.
Why not the others
Allergic to shellfish ❌ – Not relevant to transfusion risk.
Receiving blood products from relatives ❌ – Main risk here is TA-GVHD (prevented by irradiation), not anaphylaxis.
Cardiovascularly limited ❌ – This increases risk of TACO (circulatory overload), not anaphylaxis.
Shortness of breath can be a symptom of all of the following transfusion reactions except:
TRALI
TACO
Acute Hemolytic
Febrile
Febrile
Why
Febrile non-hemolytic transfusion reactions (FNHTRs) typically cause fever, chills, rigors, sometimes headache or malaise — not respiratory distress.
Shortness of breath is not a hallmark symptom unless another reaction is occurring simultaneously.
Why the others can cause SOB
TRALI – Noncardiogenic pulmonary edema → acute hypoxemia, SOB, bilateral infiltrates on CXR.
TACO – Volume overload → pulmonary edema, dyspnea, orthopnea, hypertension.
Acute Hemolytic – Severe cases can cause hypotension, shock, and respiratory distress secondary to multi-organ failure.
Which of the following transfusion reactions is associated with an increase in blood pressure?
Sepsis
TACO
TRALI
Delayed Hemolytic
TACO (Transfusion-Associated Circulatory Overload)
Why
TACO = volume overload from transfusion → hypertension (↑ BP), dyspnea, orthopnea, pulmonary edema.
Physical findings: elevated BNP, jugular venous distension, crackles on lung exam.
Why not the others
Sepsis ❌ – Usually causes hypotension from distributive shock.
TRALI ❌ – Noncardiogenic pulmonary edema → hypotension is more common than hypertension.
Delayed Hemolytic ❌ – Usually asymptomatic or mild; if symptomatic, hypotension can occur.
Which lab result would lead you to believe the blood product has been bacterially contaminated?
Positive DAT on post transfusion specimen
Positive hemolysis on the post transfusion specimen
Positive gramstain on the post patient specimen
Positive gramstain on the transfused unit
Positive gram stain on the transfused unit
Why
The most direct confirmation of bacterial contamination of a blood product is finding bacteria in the unit itselfvia Gram stain and culture.
Post-transfusion patient specimens can help confirm sepsis in the patient, but they don’t prove the source was the transfused unit.
Why not the others
Positive DAT on post-transfusion specimen ❌ – Indicates antibody-coated RBCs; not specific for bacterial contamination.
Positive hemolysis on post-transfusion specimen ❌ – Could be from many causes (immune, mechanical, thermal); not diagnostic for bacterial contamination.
Positive Gram stain on the post-patient specimen ❌ – Shows bacteremia but not necessarily that it came from the transfused product.
Which is the first step of the transfusion reaction workup?
Clerical Check
Blood Type on post specimen
DAT on the pre specimen
Gram stain of the transfused product
Clerical check
Why
In any suspected transfusion reaction, the very first step is to stop the transfusion and perform a clerical check.
This verifies that the right unit was given to the right patient and that all identifying information on the product, patient armband, and documentation match.
Clerical errors are the most common cause of acute hemolytic transfusion reactions.
Why not the others
Blood type on post specimen ❌ – Done later in the workup to check for ABO incompatibility.
DAT on the pre specimen ❌ – DAT is performed on the post-transfusion specimen to detect antibody-coated RBCs.
Gram stain of the transfused product ❌ – Done only if bacterial contamination is suspected, not as the universal first step.
Which chemical marker is expected to increase if a patient has experienced a hemolytic transfusion reaction?
Ferritin
Haptoglobin
pH
HemoglobinA1C
Ferritin… double check with Rachelle
Why
In a hemolytic transfusion reaction, RBCs are destroyed, releasing hemoglobin → broken down into iron, which increases serum ferritin (iron storage protein).
Other expected changes: ↑ plasma free hemoglobin, ↑ bilirubin, ↑ LDH, ↓ haptoglobin.
Why not the others
Haptoglobin ❌ – Actually decreases because it binds the free hemoglobin released during hemolysis.
pH ❌ – Severe reactions can cause metabolic acidosis, but pH change is not the main marker.
Hemoglobin A1C ❌ – Reflects long-term glucose control; unrelated to acute hemolysis.
A patient became hypotensive and went into shock after receiving 50mL of a unit of platelets. She had shaking and chills and her temp spiked up to 103F. A transfusion reaction investigation was initiated. Gram stain results found GPCs in the patient that matched the unit. Should this be reported to the FDA?
Yes
No
Yes
Why
This is septic transfusion reaction due to bacterial contamination of a platelet unit (Gram-positive cocci in both patient and unit).
FDA regulations require reporting of any death or life-threatening transfusion reaction that is confirmed or highly likely to be related to a blood product.
Platelets are stored at room temperature, so they’re more prone to bacterial growth than refrigerated or frozen products.
Key reporting rule
Fatal reactions → must be reported to the FDA’s CBER (Center for Biologics Evaluation and Research) within 7 days.
Even if the patient survives, confirmed bacterial contamination of a transfused component is a Biological Product Deviation and is also reportable.
A 52 yo male quadriplegic has a history of A positive with anti-Fya in his plasma. Current specimen demonstrated in ABO discrepancy in the reverse type that was attributed to a nonspecific cold agglutinin. Two units of A positive RBCS were phenotyped for Fya and found negative. They were crossmatched. The first unit was transfused with no problems, the second unit was transfused and stopped after 20 minutes when brown tinged urine was noticed in the patient’s catheter bag. Post transfusion specimen was grossly hemolyzed with a positive DAT eluate confirmed anti-E. What transfusion reaction occurred?
Acute Hemolytic
Symptoms not related to transfusion
TACO
FNHTR
Acute Hemolytic
Why
Brown-tinged urine post-transfusion = hemoglobinuria → hallmark of intravascular hemolysis.
Positive DAT with eluate identifying anti-E means the patient was transfused with E-positive blood despite having (or forming) anti-E.
This represents an acute hemolytic transfusion reaction from an incompatible unit.
Key points in the scenario
Patient had known anti-Fya, but no anti-E detected initially → likely had low/undetectable anti-E from prior sensitization (anamnestic response).
The second unit was E-positive → anti-E bound donor RBCs → rapid intravascular destruction.
Lab evidence: hemolyzed plasma, positive DAT, antibody elution showing anti-E.
Clinical evidence: hemoglobinuria, occurred within minutes of transfusion.
Why not the others
Symptoms not related to transfusion ❌ – Clear temporal link + serologic proof.
TACO ❌ – Volume overload causes respiratory distress, hypertension, pulmonary edema, not hemoglobinuria.
FNHTR ❌ – Causes fever/chills, not hemolysis or hemoglobinuria.
A 76 yo female oncology patient was in for urosepsis. Her history indicates that she is AB negative with a negative antibody screen. Her current type matches and she is crossmatched 2 units of A negative blood. Upon receiving the first blood product, she becomes hypoxic so the unit is stopped after 35mL are infused. Her blood temperature falls from 38C to 36C her BP fell from 120/80 to 110/69. Serological workup shows no evidence of clerical error or hemolysis. She died 12 hours after the transfusion reaction occurred. What type of reaction was this?
Acute Hemolytic
Symptoms not related to transfusion
TACO
FNHTR
Symptoms not related to transfusion
Why
The patient’s symptoms — hypoxia, drop in temperature (not fever), mild BP drop — don’t match the classic profile for acute hemolytic reaction, TACO, TRALI, or FNHTR.
No hemolysis was found in the workup, and the antibody screen was negative with correct crossmatch.
Death occurred 12 hours later from underlying illness (urosepsis), not a direct transfusion complication.
Key reasoning
Acute hemolytic ❌ – No evidence of ABO incompatibility or hemolysis, DAT negative.
TACO ❌ – No signs of volume overload (no hypertension, pulmonary edema, JVD).
FNHTR ❌ – No fever; temp dropped instead of rising.
The patient’s rapid decline is more consistent with progression of her sepsis, not the transfusion.
True or False: FDA is responsible for inspecting both registered and unregistered laboratories performing Transfusions.
True
False
False double check with Rachelle
Reason: The FDA inspects registered manufacturing facilities, not all transfusion-performing labs.
CBER is the department of which agency responsible for overseeing the nation’s blood supply?
FDA
CDC
AABB
JCAHO
FDA
Why
CBER = Center for Biologics Evaluation and Research
Part of the U.S. Food and Drug Administration (FDA)
Oversees:
Blood and blood components
Cellular therapies
Vaccines
Other biologics
Ensures safety, purity, potency, and efficacy of the U.S. blood supply.
Why not the others
CDC – Public health surveillance and epidemiology, not regulatory oversight of blood manufacturing.
AABB – Professional organization that sets voluntary standards and provides accreditation, but not a federal regulatory agency.
JCAHO (The Joint Commission) – Accredits hospitals and health care organizations, not the national blood supply.
Which of the following is NOT defined as a manufacturer?
Transfusion service that serves a NICU by making aliquots for neonate transfusions.
Donor collection center where apheresis platelets are collected and processed
Offsite NTL where viral testing on Donor products is performed
Courier service for blood products from the Donor Processing Center to the Transfusion Service
Courier service for blood products from the Donor Processing Center to the Transfusion Service
Why
Manufacturer (in FDA blood banking context) = any facility involved in the collection, processing, testing, storage, labeling, or distribution of blood and blood components.
Courier services simply transport products; they don’t alter, process, or test them — so they are not considered manufacturers under FDA regulations.
Why the others are manufacturers
NICU aliquots – Splitting units into aliquots is a manufacturing step under FDA.
Apheresis platelet collection center – Actively collects and processes products → manufacturing.
Offsite NTL viral testing lab – Performs infectious disease testing on donor blood → part of manufacturing process.
Which of the following Quality Assurance activities should NOT be performed by the manufacturer?
Inspection of Transfusion Services written SOPs
Product purity and potency Quality Control
Label inspection for license
Short Supply Arrangements
Inspection of Transfusion Services written SOPs
Why
Manufacturers (FDA-registered blood collection/processing facilities) are responsible for QA activities within their own operations — e.g., product purity/potency QC, label control, licensing compliance, and managing short supply arrangements.
Inspecting a hospital transfusion service’s SOPs is not a manufacturer’s responsibility — that falls under accrediting bodies (AABB, CAP, Joint Commission) or the hospital’s own QA program.
Why the others are manufacturer QA responsibilities
Product purity and potency QC – Ensures products meet standards before release.
Label inspection for license – Part of CGMP to ensure correct labeling per FDA license.
Short supply arrangements – Documented procedures for distributing limited products safely.
How long should Donor deferral records be maintained by the Blood Donor Center?
2 years
5 years
10 years
Indefinitely
10 Years
Why
FDA regulations (21 CFR 606.160) require donor deferral records to be maintained for 10 years from the date of deferral.
This ensures that any future donations from the same person can be evaluated against prior deferral history, especially for permanent deferrals (e.g., positive infectious disease tests).
Other retention times for context
Routine donor & product records – At least 10 years
Certain QC & manufacturing records – 5 years minimum
Transfusion service patient records – Often 10 years (per AABB) but can vary by state law
A platelet is returned from surgery 1 hour after issue unused on ice, what do you do?
Discard, it has been out of the blood bank too long
Discard, it has been stored improperly
Return to inventory, platelets are fine up to 24 hours
Return to inventory, platelets are rare and must be preserved until expiration
Discard, it has been stored improperly
Why
Platelets must be stored at 20–24 °C with continuous gentle agitation to maintain function and prevent bacterial growth.
Putting platelets on ice damages them irreversibly — cold storage causes platelet activation and loss of viability.
Even though the time out of the blood bank was only 1 hour, improper storage temperature makes them unusable.
Why not the others
“Too long” ❌ – Time isn’t the problem here; temp is.
“Fine up to 24 hours” ❌ – Only if stored properly at room temp with agitation.
“Rare and must be preserved” ❌ – Safety/quality standards override rarity; improperly stored components must be discarded.
Which of the following tests counts as QC not QA
Lookback activities
Direct observation of test performance by a seasoned tech
Parallel testing of kit components from a new Fetal Screen Kit
Validation of a new automated blood banking analyzer
Direct observation of test performance by a seasoned tech
Reasoning (per lecture):
QC = operational checks done in real-time to ensure a test, reagent, or instrument is functioning properly at the time of use (e.g., control samples, observing technique).
QA = broader program of oversight, documentation, validation, and policy review to ensure quality over time.
The other options are QA activities:
Lookback activities → QA follow-up process.
Parallel testing of kit components → QA validation of new reagent lots.
Validation of new analyzer → QA validation process.Blood Bank Examples
QC (real-time, test-specific)
Running positive/negative controls on a new antibody panel lot
Parallel testing of a new fetal screen kit
Checking thermometer calibration before releasing blood
Daily review of centrifuge RPMs
QA (big picture, ongoing system oversight)
Annual competency observation of a tech
Lookback investigation after a donor’s post-donation positive test
Validation of a new analyzer
Policy review for RhIg administration
Which Quality Assurance activity is considered process improvement?
Competency Performance for a new employee
Lookback Activities
Update of SOPs
Quarterly Review of Preventive maintenance records
Update of SOPs
Why
Process improvement in QA = changing policies, procedures, or workflows to enhance safety, efficiency, or compliance.
Updating Standard Operating Procedures (SOPs) after identifying a better or safer method is a direct example of process improvement.
Why not the others
Competency Performance for a new employee ❌ – QA activity for personnel assessment, not process improvement.
Lookback Activities ❌ – QA activity for traceability and safety follow-up, not changing the process.
Quarterly Review of Preventive Maintenance records ❌ – QA monitoring/oversight, not improvement.
According to Patient Blood Management Guidelines what is the transfusion Threshold for a patient about to undergo cardiothoracic surgery?
6 g/dL
6-7 g/dL
8-10 g/dL
>10 g/dL
8–10 g/dL
Reasoning:
For high-risk surgeries like cardiothoracic procedures, maintaining higher preoperative hemoglobin is recommended to ensure adequate oxygen delivery during and after surgery.
This threshold is higher than the restrictive 7 g/dL used for stable, non-bleeding patients because of the increased physiologic demand and risk of ischemia
According to Patient blood management guidelines, what test should be ordered between transfusions of RBCs?
Blood Type
Bilirubin
Hemoglobin
INR
Hemoglobin
Reasoning:
PBM emphasizes avoiding unnecessary transfusion and using hemoglobin measurement between units to assess whether the patient still meets transfusion criteria.
This prevents over-transfusion, reduces risks, and supports evidence-based blood utilization.
Who manages physician education regarding patient blood management activities?
The Blood Bank Staff
The Blood Bank Manager
The Laboratory Director
Other physicians
The Laboratory Director
Reasoning:
Per Patient Blood Management (PBM) and compliance standards, the Laboratory Director holds ultimate responsibility for ensuring physicians are educated about PBM principles, transfusion guidelines, and appropriate ordering practices.
The blood bank staff/manager may assist with data collection and in-service training, but regulatory oversight assigns this responsibility to the director.
What is an expected outcome of a patient blood management program implementation at a hospital?
The hospital will pass JCAHO inspection
Better patient outcomes from lower transfusion thresholds
Better interaction amongst transfusion staff and physicians
More reimbursement from CMS
Better patient outcomes from lower transfusion thresholds — PBM programs are designed to optimize transfusion practices, reducing unnecessary transfusions and improving morbidity/mortality rates.
Why the others are wrong:
The hospital will pass JCAHO inspection — Accreditation isn’t the primary goal; improved care is.
Better interaction amongst transfusion staff and physicians — Possible side benefit, but not the main measured outcome.
More reimbursement from CMS — PBM may lower costs, but reimbursement is not the direct aim.
Which patient is matched correctly with the crossmatch they should be getting?
B pos with 2 ABO types that match and an anti-E ; Electronic
A neg with no previous history and a negative antibody screen; Immediate spin
O positive with 2 ABO types that match and negative antibody history; AHG
A neg with no previous history and a negative antibody screen; Immediate spin – Immediate spin crossmatch is appropriate for first-time patients with no antibody history and a negative antibody screen.
Why Others Are Incorrect:
B pos with 2 ABO types that match and an anti-E; Electronic – Electronic crossmatch is only allowed if there is no clinically significant antibody history; anti-E requires serologic (AHG) crossmatch.
O positive with 2 ABO types that match and negative antibody history; AHG – AHG crossmatch is unnecessary without antibody history or current positive screen; immediate spin or electronic would suffice.

Which follow up test might you perform for a multiple myeloma patient with the following results?

Wash cells
PreWarm Technique
Saline Replacement
Cool to 4C
Saline replacement — Multiple myeloma causes rouleaux from excess proteins, giving spurious agglutination in reverse typing; saline replacement disperses rouleaux and resolves the ABO discrepancy.
Why the others are wrong (very short):
Wash cells — Helps forward-type issues, not reverse rouleaux.
Prewarm technique — For cold autoantibodies, not protein-induced rouleaux.
Cool to 4 °C — Enhances cold agglutinins; would worsen, not fix, this pattern.
Your patient is A negative and has anti-Fyb in their plasma. 2 units of RBCs are ordered for a morning surgery. You have tested all 3 A negative units in inventory for the antigen and they are all positive. What do you do next?
Crossmatch 2 of the A negative units that you phenotyped
Phenotype the O negative units in inventory for Fyb antigen
Crossmatch 2 O negative units in inventory for the patient
Phenotype the AB negative units in inventory for Fyb antigen
Cancel the surgery
Phenotype the O negative units in inventory for Fyb antigen — You need Fyb-negative RBCs; O⁻ is ABO/Rh compatible with an A⁻ patient, so phenotype O⁻ units to find Fyb-negative ones for crossmatch.
Why the others are wrong (very short):
Crossmatch A⁻ units you phenotyped — They’re Fyb positive; will be incompatible.
Crossmatch 2 O⁻ units — Don’t skip antigen typing; many O⁻ units are Fyb positive.
Phenotype AB⁻ units — AB RBCs are ABO incompatible for an A patient.
Cancel the surgery — Premature; first obtain Fyb-negative O⁻ units or request from supplier.
Which surgical patient would require the use of irradiated blood products?
Jehovah’s witness heart surgery patient
7th day Adventist orthopedic patient
AIDS patient undergoing kidney transplant
5 year old sickle cell patient undergoing splenectomy
AIDS patient undergoing kidney transplant — Irradiation prevents TA-GVHD in immunocompromised patients (e.g., AIDS, transplant recipients).
Why the others are wrong (very short):
Jehovah’s witness heart surgery — Likely declines transfusion; irradiation not specifically indicated.
7th day Adventist orthopedic — No transfusion modification required solely for religion.
5-year-old sickle cell splenectomy — Needs antigen-matched blood, not irradiation, unless also immunocompromised.
Which procedure is not utilized in bloodless medicine?
Plasmapheresis
Cell Salvage
Hemodilution
Vitamin K administration
Plasmapheresis — This removes plasma (and potentially needed components) rather than conserving or minimizing blood loss; it is not a standard bloodless medicine technique.
Why the others are wrong (very short):
Cell Salvage — Conserves patient’s own blood during surgery.
Hemodilution — Dilutes patient’s blood pre-op to reduce RBC loss during surgery.
Vitamin K administration — Supports coagulation and reduces bleeding risk.
A patient has a history of A positive blood type. The current sample is O positive. What should be done regarding transfusion?
Crossmatch O negative blood to the current sample.
Have the patient recollected STAT.
Change the record to match the current blood type.
Offer Emergency release O negative blood.
Have the patient recollected STAT — The discrepancy must be resolved before transfusion; recollection ensures no clerical or sample error.
Why the others are wrong (very short):
Crossmatch O negative blood to the current sample — Risks giving wrong type if current sample is wrong.
Change the record to match the current blood type — Never change without confirmation.
Offer Emergency release O negative blood — Only if patient is actively bleeding and transfusion cannot wait for resolution.
Which is the TRUE statement?
Emergency releases are always massive transfusions.
Massive transfusions always have emergency releases.
Both emergency release and massive transfusion wave the necessity of performing a crossmatch on a patient.
A crossmatch must complete the workup for both emergency release and massive transfusions.
A crossmatch must complete the workup for both emergency release and massive transfusions — Even if blood is issued before compatibility testing, the crossmatch must be performed as soon as possible afterward.
Why the others are wrong (very short):
Emergency releases are always massive transfusions — Not true; emergency release can be for small volume.
Massive transfusions always have emergency releases — Not always; many are planned and matched in advance.
Both emergency release and massive transfusion waive the necessity of performing a crossmatch — They only delay it; crossmatch is still required later.
Plasma transfusion instead of cellular products would be indicated for which of the following patients?
Sickle cell patient not in crisis
TTP patient
Warm Autoantibody patient
A mother at risk of causing HDFN
All of the above
None of the above
TTP patient — Plasma is used in plasma exchange for TTP to replace ADAMTS13 and remove inhibitory antibodies.
Why the others are wrong (very short):
Sickle cell patient not in crisis — Would need RBCs, not plasma.
Warm Autoantibody patient — Managed with compatible RBC transfusion if anemic.
Mother at risk of causing HDFN — RhIG is indicated, not plasma.
All of the above / None of the above — Only TTP is correct.
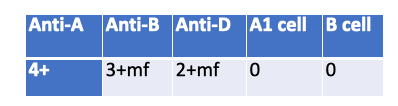
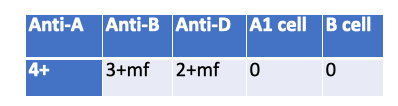
What is a possible explanation for the following serological results?
Multiple Myeloma
WAIHA
Immunocompromized
Massive Transfusion
Massive Transfusion — Mixed-field agglutination with Anti-B (3+ mf) and Anti-D (2+ mf) plus absent reverse reactions (0/0) fits recent heavy transfusion: two RBC populations present and patient antibodies diluted/supplied by donor plasma.
Why the others are wrong (very short):
Multiple Myeloma — Causes rouleaux → spurious positive reverse typing, not 0/0, and no mixed-field in forward.
WAIHA — Panagglutination/DAT issues; not the clean mixed-field pattern in specific forward reagents.
Immunocompromized — Can explain weak/absent reverse (0/0) but doesn’t produce mixed-field in forward typing.
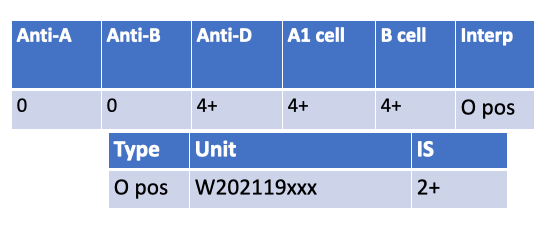
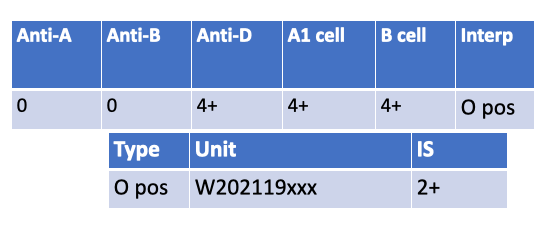
What is the most likely possible explanation for the following serological results?
CAD
Pos DAT in Donor cell
Pos DAT in patient cells
Low incidence antibody
CAD (cold agglutinin disease) double check with Rachelle— An IS crossmatch (2+) despite ABO-identical O⁺→O⁺ most often points to a cold-reactive IgM autoantibody in the patient’s plasma causing room-temp agglutination.
Why the others are wrong (very short):
Pos DAT in donor cell — DAT-positive donor cells cause issues at AHG, not typically at immediate spin (IgG coating doesn’t agglutinate without AHG).
Pos DAT in patient cells — Patient DAT status doesn’t make their plasma vs donor RBC IS crossmatch incompatible.
Low incidence antibody — Usually shows single-unit incompatibility and at AHG phase, not broad IS reactivity.
Which of the following is acceptable as a donor?
29 yo who received flu vacc last month.
21 yo who got nose pierced last week.
30 yo who used to live in Zambia and returned last year.
54 yo tested pos for HepC last year but no active symptoms
29 yo who received flu vacc last month — Flu vaccines (non-live or live-attenuated) have no deferral for blood donation if donor is well.
Why the others are wrong (very short):
Nose pierced last week — 3–12 mo deferral (infection risk, esp. if non-sterile technique).
Lived in Zambia — 3 yr deferral for malaria-endemic residence after return.
Hep C positive last year — Permanent deferral (risk of transmission).
Which vaccine has the longest deferral period?
Hep B Ig
Rubella
Influenza
Yellow fever
Hep B Ig — 12-month deferral due to potential HBV exposure.
Others (short reason):
Rubella — 4-week deferral.
Influenza — No deferral if donor is healthy.
Yellow fever — 2-week deferral.
Which of the following donors are qualified to donate on September 10?
40 yo who donated RBCs on July 23
28 yo who donated platelets on Aug 24
52 yo who made an autologous donation 2 days ago
23 yo who donated blood for her aunt on Aug 14
28 yo who donated platelets on Aug 24
Reasoning:
Platelet donation interval is ≥ 48 hours (up to 24 times/year), so Aug 24 → Sep 10 is fine.
Why others are wrong:
40 yo who donated RBCs on July 23 → Whole blood/RBC interval is 56 days; July 23 → Sep 10 is only 49 days
52 yo who made an autologous donation 2 days ago → Must wait ≥ 3 days before another donation
23 yo who donated for her aunt on Aug 14 → Directed donations follow same interval rules as whole blood (56 days); Aug 14 → Sep 10 is only 27 days
Which donor would be acceptable for whole blood donation?
Former drug addict who has been clean for 3 years
Triathlete with a pulse of 45 beats/min
A man who is currently in remission for multiple myeloma
A woman treated for gonorrhea 8 months ago
Triathlete with a pulse of 45 beats/min
Reasoning:
Athletes can have low resting heart rates (<50 bpm) without pathology — acceptable if asymptomatic and otherwise meets criteria.
Why others are wrong:
Former drug addict who has been clean for 3 years → Permanent deferral for history of IV drug use
Man in remission for multiple myeloma → Permanent deferral for hematologic malignancy
Woman treated for gonorrhea 8 months ago → 12-month deferral after treatment for high-risk STD; only 8 months passed
Which physical exam result is cause for rejecting a whole blood donor?
Weight 105 lbs
Pulse 75 beats/min
Temperature of 99.3F
Diastolic blood pressure of 110 mm Hg
Diastolic blood pressure of 110 mm Hg
Reasoning:
Acceptable diastolic BP for donation is generally ≤100 mm Hg; 110 mm Hg exceeds the limit and is cause for deferral.
Why others are wrong:
Weight 105 lbs → Meets minimum weight requirement (≥110 lbs is required in some settings, but AABB standard for standard volume donation is 110 lbs; 105 lbs would be cause for deferral if standard volume is drawn—need confirmation of volume drawn).
Pulse 75 bpm → Within normal donor range (50–100 bpm, or lower if athletic).
Temperature 99.3 °F → Below deferral threshold (≥99.5 °F).
Which donor is acceptable for blood donation today?
Donor who had an abortion 4 weeks ago
Donor who’s husband is a hemophiliac who received cryo in the 1980s
Donor treated for gonorrhea 6 months ago
Donor who had a needlestick 9 months ago
Donor who had an abortion 4 weeks ago
Reasoning:
Abortion or miscarriage is typically acceptable after recovery, with no minimum deferral period per AABB, as long as donor meets all other eligibility criteria.
Why others are wrong:
Husband is a hemophiliac who received cryo in the 1980s → Permanent deferral due to potential exposure risk for HIV from products made before screening.
Treated for gonorrhea 6 months ago → 12-month deferral after treatment per AABB infectious disease guidelines.
Needlestick 9 months ago → 12-month deferral due to possible exposure to bloodborne pathogens.
Which test is NOT required for allogenic donation testing?
Rh
STS
Anti-HTLV
Anti-CMV
Anti-CMV
Reasoning:
Anti-CMV is not required for allogeneic donation testing; it is only performed when CMV-negative products are specifically requested for at-risk recipients.
Why others are wrong:
Rh → Required for donor typing.
STS → Syphilis testing (Serologic Test for Syphilis) is required.
Anti-HTLV → Required to screen for Human T-cell Lymphotropic Virus I/II.
What is the difference between allogenic donor and autologous donor?
There is not a minimum age for autologous donors.
Hemoglobin value has a lower threshold for autologous than allogenic
The autologous donor unit will not be rejected for viral testing results.
All of the above
All of the above
Reasoning:
No minimum age → Autologous donations are for self-use; age restrictions are flexible if physician approves.
Lower hemoglobin threshold → Acceptable because risk of transfusion reaction is low when transfusing back to self.
Viral testing → Positive results do not result in discard, as the unit is returned to the same donor-patient.
Which of the following lists the correct shelf life for the product?
Deglycerolized RBCs- 24 hours
CPD RBCs – 35 days
Platelet concentrate – 7 days
FFP – 5 years
Deglycerolized RBCs – 24 hours
Reasoning:
Deglycerolized RBCs → Washed product; must be used within 24 hours once thawed due to contamination risk.
Why others are wrong:
CPD RBCs – 35 days → Incorrect; CPD RBCs expire in 21 days.
Platelet concentrate – 7 days → Incorrect; shelf life is 5 days at 20–24°C with agitation (some FDA-approved systems allow 7, but standard is 5).
FFP – 5 years → Incorrect; frozen at ≤ –18°C lasts 1 year; at ≤ –65°C lasts 7 years.
What cells are HLA antigens typically on?
Red cells
White cells
Platelets
2 of these
2 of these
Reasoning:
HLA antigens are typically found on white cells (WBCs) and platelets.
Why others are wrong:
Red cells → Do not typically express HLA antigens (except weakly with some residual WBC contamination).
White cells → Correct, they express abundant HLA antigens.
Platelets → Correct, they express class I HLA antigens.
What is the test of choice for HLA antigen testing?
Agglutination
Molecular
Cytotoxicity
ELISA
Molecular
Reasoning:
Molecular methods (e.g., PCR-based typing) are now the gold standard for HLA antigen testing due to higher resolution, accuracy, and ability to distinguish allelic variants.
Why others are wrong:
Agglutination → Not used for HLA testing; suited for ABO/Rh typing.
Cytotoxicity → Older method (complement-dependent cytotoxicity assay); less precise and used mainly historically.
ELISA → Can detect HLA antibodies, not optimal for antigen typing.
Which coagulation factors are diminished in products labeled “thawed plasma”
XIII & VWF
V & VIII
Fibrinogen & VIII
VII & Fibrinogen
V & VIII
Reasoning:
In thawed plasma, Factor V and Factor VIII decrease most rapidly after thawing because they are labile factors sensitive to storage.
Why others are wrong:
XIII & VWF → Stable in thawed plasma.
Fibrinogen & VIII → Fibrinogen remains stable; VIII does drop, but fibrinogen is not significantly affected.
VII & Fibrinogen → Both are relatively stable in thawed plasma.
Which of the following is true regarding apheresis platelets?
Minimum platelet count in a unit must be 3.0 x 10 11 PH<6.0
Minimum platelet count in a unit must be 3.0 x 10 10 PH<6.2
Minimum platelet count in a unit must be 3.0 x 10 11 PH>6.2
Minimum platelet count in a unit must be 5.5 x 10 10 PH>6.0
Minimum platelet count in a unit must be 3.0 × 10¹¹, pH > 6.2
Reasoning:
FDA and AABB standards require apheresis platelet units to contain ≥3.0 × 10¹¹ platelets and to have a pH > 6.2 at the end of the storage period to maintain viability and function.
Why others are wrong:
3.0 × 10¹¹, pH < 6.0 → pH is too low; platelets lose viability.
3.0 × 10¹⁰, pH < 6.2 → Platelet count is far too low; unit wouldn’t meet therapeutic dose.
5.5 × 10¹⁰, pH > 6.0 → That’s the standard for a single platelet concentrate from whole blood, not an apheresis unit.
A shipment of packed red blood cells and platelets arrived together in the same box stored at 1-6C. What should be done?’
Place all units in 1-6C refrigerator.
Reject the entire shipment.
Accept the red cells, discard the platelets.
Accept the platelets, discard the red cells.
Accept the red cells, discard the platelets.
Reasoning:
RBCs are stored at 1–6 °C, so they are fine.
Platelets must be stored at 20–24 °C with continuous gentle agitation; exposure to refrigeration irreversibly damages them.
Why others are wrong:
Place all units in 1–6 °C refrigerator → Would destroy the platelets.
Reject the entire shipment → Unnecessary; RBCs are still usable.
Accept the platelets, discard the red cells → Platelets are already compromised from cold storage.
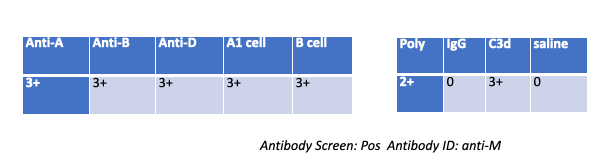
A patient gives the following results on work up, what followup testing should you do?
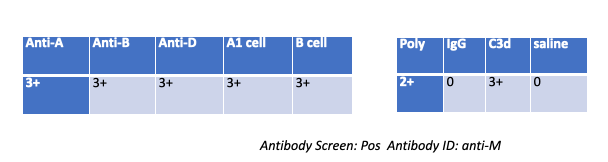
Wash patient cells
Eluate
Adsorption
Nothing
Nothing — The DAT is C3d-positive, IgG-negative and the antibody ID is anti-M (cold, usually IgM). With complement-only coating, an eluate (for IgG) won’t yield anything, and no adsorption is indicated.
Why the others are wrong (very short):
Wash patient cells — Routine step already done; not a specific follow-up.
Eluate — Recovers IgG antibodies; DAT shows no IgG.
Adsorption — Not needed for a cold IgM anti-M with complement-only DAT.