intermolecular forces
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
Intramolecular Forces
The attractive forces between atoms and ions within a molecule
relatively strong
eg — ionic/covalent bond
Intermolecular forces
attractive forces between molecules
weak in comparison to intramolecular forces
eg — dipole-dipole, London dispersion, hydrogen bonds
how does intermolecular forces determine physical properties (state, melting/boiling point, hardness/texture, solubility)?
the stronger the IMFs in the sample of molecules, the more strongly they interact, which means they stick together more. That leads to the following trends: Stronger IMF → Higher melting and boiling points (harder to melt and boil) Stronger IMF → Lower vapor pressure (harder to boil)
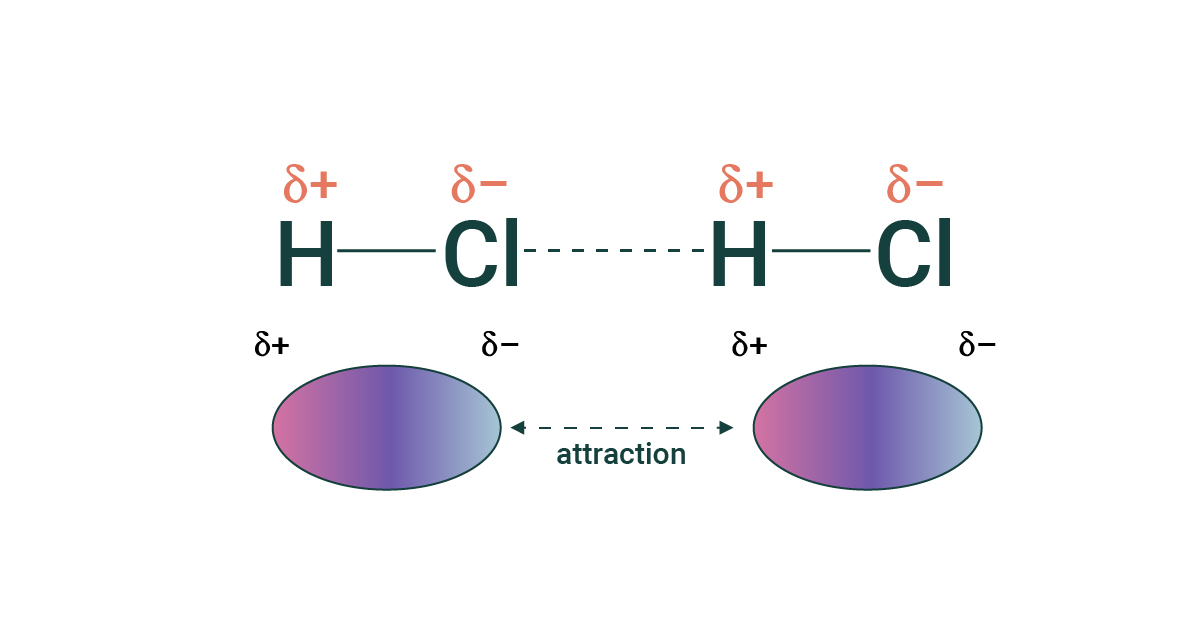
Dipole-Dipole
Forces of attraction between oppositely charged ends of polar molecules.
relatively strong IMF
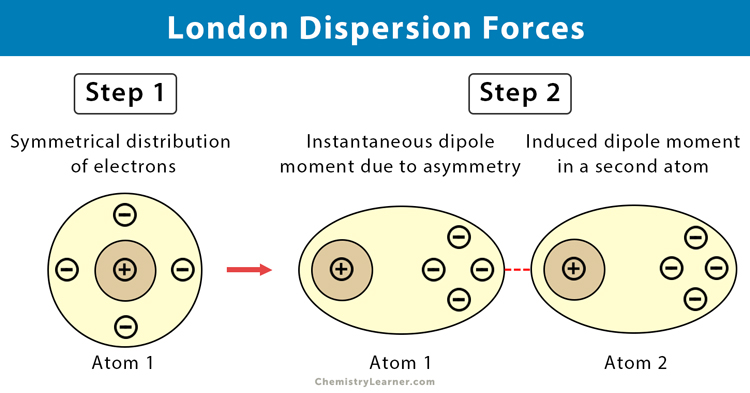
London Dispersion
Attractive forces between all molecules, including nonpolar molecules
Result of temporary displacements of the electron cloud around atoms in a molecule (extremely short-lived dipoles)
thus weaker than dipole-dipole
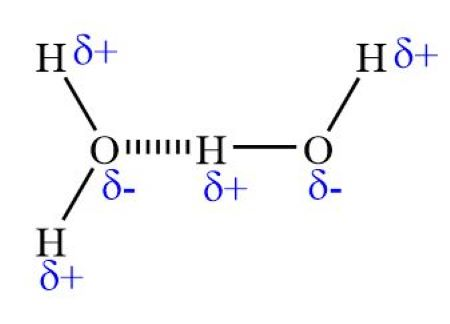
Hydrogen Bonding
Strong dipole-dipole force between the positive hydrogen atom of one molecule and highly electronegative atom of another molecule (O, N, F)