Biochem Lec 23- Glycolysis Regulation and Gluconeogenesis
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
How many steps are considered irreversible in glycolysis (under cellular conditions)? What are they?
Three: Reactions 1,3, and 10
Reaction 1→ Hexokinase
Glucose + ATP→ Glucose-6-phosphate (G-6P) + ADP + H+
Reaction 3→ Phosphofructokinase (PFK)
Fructose-6-phosphate + ATP→ Fructose-1,6-bisphosphate (F-1,6-BP) + ADP + H+
Reaction 10→ Pyruvate Kinase
Phosphoenolpyruvate→ Pyruvate
ALL have a negative free energy and are points of regulation
How does glycolysis regulation differ in muscle vs. liver?
Muscle:
Rapid production of ATP during intense exercise/exertion
Regulation is done by energy charge and the concentration of ATP vs. concentration of AMP
Liver:
Glycolysis is used to provide carbon skeletons for biosynthesis
Balance between gluconeogenesis and blood glucose during fasting
Hormonal regulation is important
What is the key step of glycolysis regulation in muscle?
Phosphofructokinase (PFK):
Committed step in glycolysis
ATP: high energy→ - allosteric effector
ATP binds a regulatory site and reduces PFK’s affinity for Fructose-6-phosphate→ prevents wasteful use of glucose at rest
AMP: Low energy→ + allosteric effector
During exercise, adenylate kinase makes AMP→ Rising AMP levels overrides ATP inhibition→ restores high affinity of PFK for F-6P and accelerates glycolysis to make ATP
Describe the structure of PFK. What is it and how does the concentration of ATP vs. AMP impact its activity (i.e., its kinetics)?
PFK is a classical allosteric enzyme:
Tetramer
Has 2 ATP binding sites
Active site→ high affinity
Allosteric binding site→ lower affinity
High [AMP] and low [ATP]→ increases reaction velocity at lower [Fructose-6-phosphate] and is hyperbolic in shape
Low [AMP] and high [ATP]→ decreases reaction velocity at lower [Fructose-6-phosphate] and is sigmoidal in shape
![<p>PFK is a classical allosteric enzyme:</p><ul><li><p>Tetramer</p></li></ul><ul><li><p>Has 2 ATP binding sites</p></li></ul><ol><li><p>Active site→ high affinity</p></li><li><p>Allosteric binding site→ lower affinity</p></li></ol><p>High [AMP] and low [ATP]→ increases reaction velocity at lower [Fructose-6-phosphate] and is hyperbolic in shape</p><p>Low [AMP] and high [ATP]→ decreases reaction velocity at lower [Fructose-6-phosphate] and is sigmoidal in shape</p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/d3b0eeec-48a6-4457-b59a-d0e47d93cb13.png)
How do the concentrations of ATP and AMP compare in resting muscle? How does this impact glycolysis?
In resting muscle, [ATP] > [AMP]:
PFK activity is inhibited→ ATP is a negative allosteric regulator of PFK
Pyruvate kinase activity is inhibited→ ATP is also a negative allosteric regulator of pyruvate kinase
PFK inhibition leads to accumulation of glucose-6-phosphate and feedback inhibits hexokinase:
Fructose-6-phosphate is not being converted by PFK→ increase in [F-6-P] shifts equilibrium of G-6-P→ F-6-P to favor the reactants and causes a build up of G-6-P
Build up of G-6-P inhibits hexokinase by shifting the equilibrium of Glucose→ G-6-P to favor the reactants and prevents further phosphorylation of glucose
High [ATP]/low [AMP] inhibits glycolysis at multiple steps (PFK, pyruvate kinase, and hexokinase indirectly) through allosteric and feedback inhibition→ glucose is not “burned”
How do the concentrations of ATP and AMP compare in working muscle? How does this impact glycolysis?
In working muscle [ATP] < [AMP]:
PFK activity is stimulated→ AMP is pos. allosteric effector of PFK→ More fructose-1,6-bisphosphate is synthesized
Pyruvate kinase is allosterically activated by fructose-1,6-bisphosphate (feed forward stimulation)→ ensures that as glycolysis speeds up, phosphoenolpyruvate is efficiently converted to pyruvate→ maintains high ATP output
Because of PFK stimulation, glucose-6-phosphate moves through glycolysis and hexokinase is active
Low [ATP]/high [AMP] stimulates glycolysis at multiple steps→ glucose is “burned” to generate ATP for muscle contraction
Describe the importance of maintaining blood glucose.
Human body requires 160 g of glucose per day for survival
120 g are required for brain function→ brain only uses glucose as fuel whereas most other tissues can use fats and other fuels
During fasting (no consumption of carbohydrate fuels), we have resources (mostly from liver and muscle glycogen) for about 200 g of glucose
During sustained starvation, muscle protein is sacrificed and amino acids are used to generate pyruvate→ converted to glucose in liver
This process is referred to as gluconeogenesis
What poses a thermodynamic problem for gluconeogenesis?
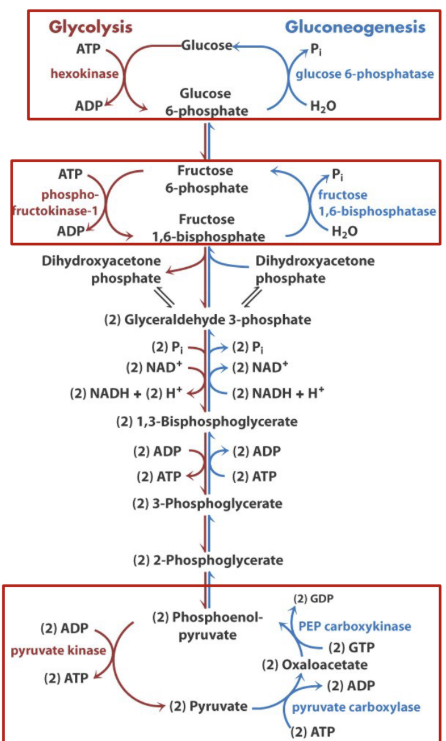
Glycolysis is irreversible:
Seven reactions of glycolysis are freely reversible
Reactions catalyzed by Hexokinase (1), PFK (3), and Pyruvate Kinase (10) are not
Four specific Gluconeogenesis enzymes are used to reverse these three steps
This process is costly: 6 high energy phosphates are required to convert pyruvate to glucose
Reversing glycolysis as a means of generating glucose is energetically costly
What is gluconeogenesis? Briefly describe it.
Gluconeogenesis→ synthesis of glucose from non-carbohydrate precursors:
Done primarily in liver
Provides glucose for bloodstream during fasting
Not exactly a reversal of glycolysis
Four reactions are needed to bypass the three irreversible steps of glycolysis
This pathway costs energy→ 6 high energy phosphates per glucose formed
What two enzymes are required to catalyze the conversion of pyruvate to phosphoenolpyruvate (reverse reaction 10)?
Pyruvate carboxylase and phosphoenolpyruvate (PEP) carboxykinase
Describe the role of pyruvate carboxylase in the conversion of pyruvate to phosphoenolpyruvate.
Catalyzes the carboxylation of pyruvate to oxaloacetate
Requires biotin→ serves as a covalently attached prosthetic group
This biotin carries an “activated CO2”:
Biotin is covalently attached to a lysine on a flexible domain (BCCP→ biotin carboxyl carrier protein)
Long and flexible and moves between two catalytic domains in pyruvate carboxylase
Biotin carboxylase (BC)
Biotin + ATP + HCO3- → Carboxybiotin + ADP + Pi
Carboxyl transferase (CT)
Carboxybiotin + pyruvate→ Biotin + Oxaloacetate
This step REQUIRES ATP
Pyruvate + CO2 + ATP + H2O→ Oxaloacetate + ADP + Pi + 2H+
Describe the structure of pyruvate carboxylase and how it relates to biotin.
Pyruvate carboxylase is a complex enzyme (1154 amino acids) that has multiple domains
The two enzymatic domains are the biotin carboxylase (BC) and the carboxyl transferase (CT) domains→ the each have their own active site
The biotin carboxyl carrier domain (BCCP) has a biotin co-enzyme attached to a lysine residue
BCCP and biotin swing between the BC and CT active sites
PT is the protein tetramerization domain which is not part of catalysis
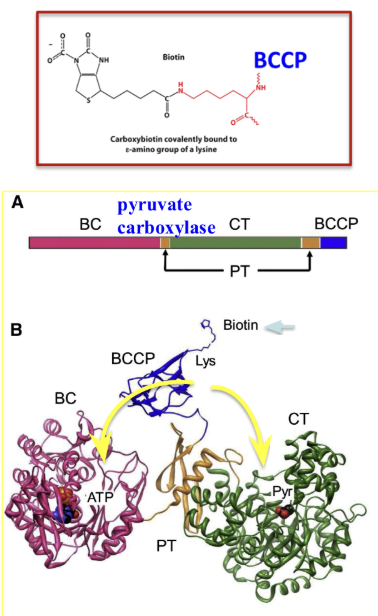
Describe the role of phosphoenolpyruvate carboxykinase in the conversion of pyruvate to phosphoenolpyruvate.
Oxaloacetate is decarboxylated and phosphorylated to generate phosphoenolpyruvate
Carboxylation/decarboxylation is a way around having to phosphorylate pyruvate directly
Oxaloacetate + GTP→ Phosphoenolpyruvate + GDP + CO2
What is the sum of “bypass” reactions?
Pyruvate + ATP + GTP + H2O→ Phosphoenolpyruvate + ADP + GDP + Pi + 2H+
Only “irreversible” steps need to be bypassed by new steps. Why is this?
Non-irreversible steps are near equilibrium under cellular conditions, so the pathway can readily run in reverse
What enzyme reverses the third reaction in glycolysis (catalyzed by PFK)?
Fructose-1,6-bisphosphatase:
Fructose-1,6-bisphosphate + H2O→ Fructose-6-phosphate + Pi
Removes a phosphate from the 1 position
What enzyme reverses the first reaction in glycolysis (catalyzed by hexokinase)?
Glucose-6-phosphatase:
Glucose-6-phosphate + H2O→ Glucose + Pi
Removes a phosphate from the 6 position
In most tissues, free glucose is not generated and glucose-6-phosphate (which cannot be transported out of the cell) is processed in some other way→ e.g., to form glycogen
Explain gluconeogenesis vs. “reverse glycolysis”
In gluconeogenesis, 6 high-transfer-potential phosphoryl groups are spent to synthesize glucose from pyruvate:
4 ATP
2 GTP
2 are gained through glycolysis (2 ATP)→ the 4 extra (2 ATP and 2 GTP) are required to turn an unfavorable reaction into a favorable one
Reverse glycolysis:
2 Pyruvate + 2 ATP + NADH + 2 H2O→ Glucose + 2 ADP + 2 Pi + 2 NAD+ +2H+
ΔGo’= +90 kJ/mol
Gluconeogenesis:
2 Pyruvate + 4 ATP + 2 GTP + 2 NADH + 6 H2O→ Glucose + 4 ADP + 2 GDP + 6 Pi + 2 NAD+ + 2H+
ΔGo’= -48 kJ/mol
Recap glycolysis and gluconeogenesis.
The reactions and enzymes involved differ at a few specific points
These points are the “irreversible” steps of glycolysis (have large negative ΔGo’) that require specific enzymes to bypass
This requires energy (4 ATP and 2 GTP per glucose)
Key “irreversible” steps are control points for regulation
Image:
Red→ distinctive reactions that differ from glycolysis. The enzymes are located in the cytosol except for pyruvate carboxylase which is in the mitochondria