PCHEM Exam 1
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
No gas is an ideal gas because there are two major assumptions that we make for ideal gases. those two are…
the particles have essentially no volume
there are no intermolecular forces
A system that allows for the transfer of neither energy nor mass is called a blank system
isolated
which of the following is considered a path function
heat
van der Waals gas
(P+ a n²/v²) (V-nb) = nRT

195 atm
state the first law of thermodynamics as an equation
ΔU = q + w
State the first law of thermodynamics in a sentence
Energy can’t be created or destroyed, only transformed or transferred
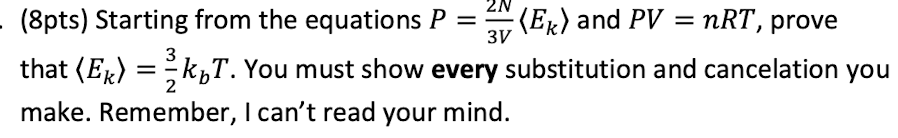
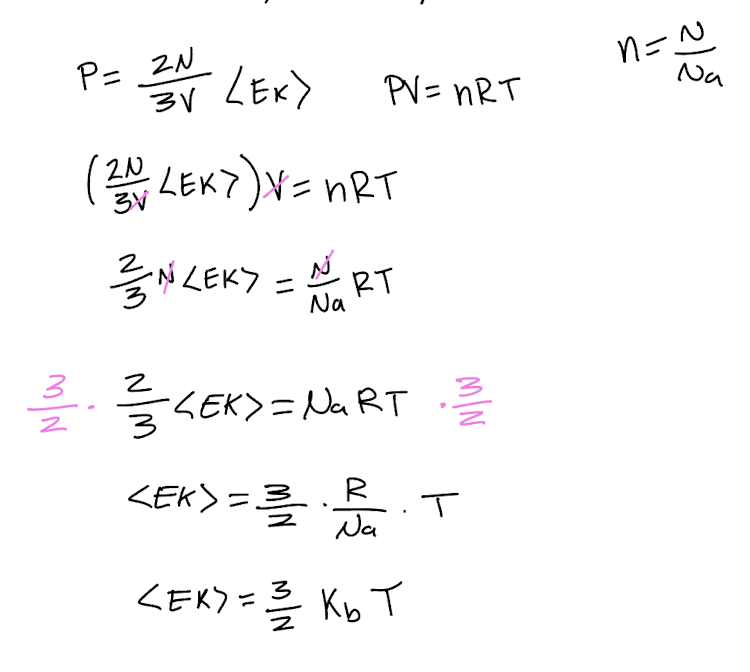

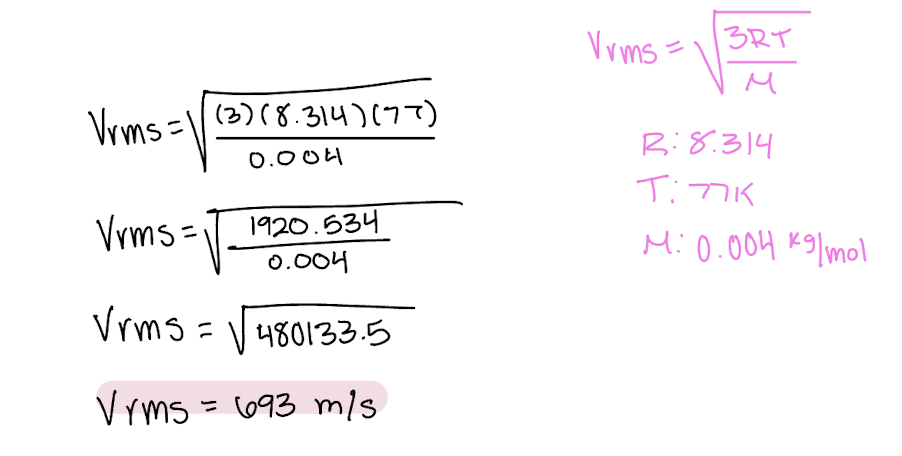
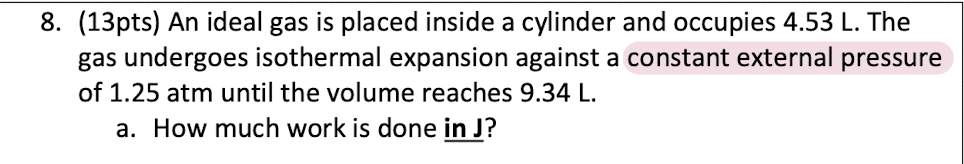
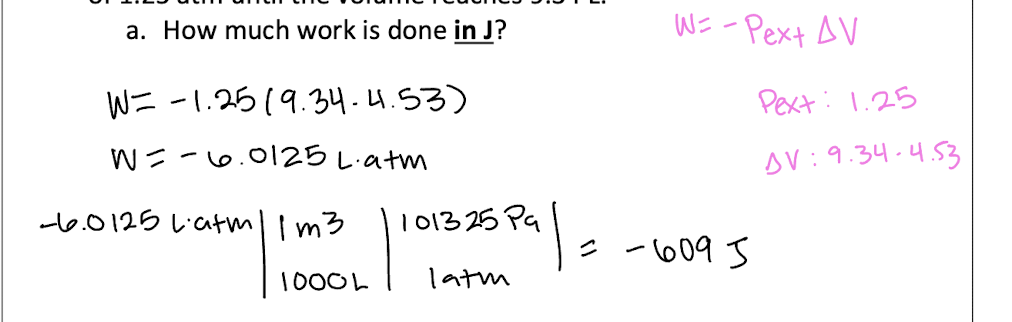

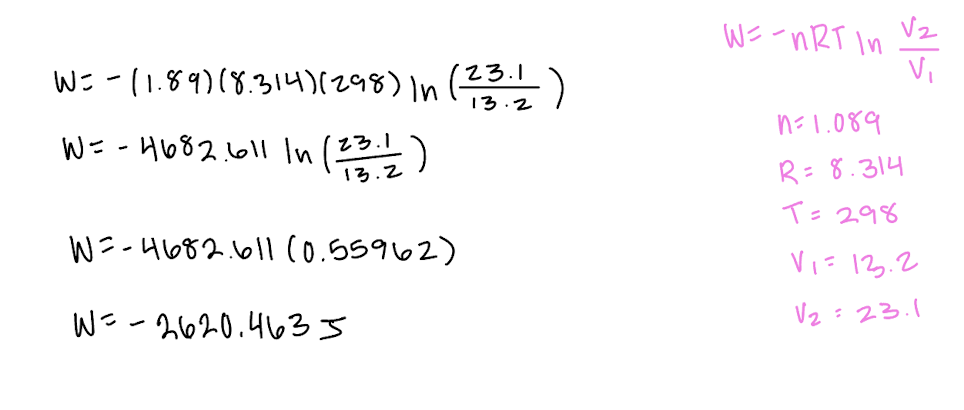

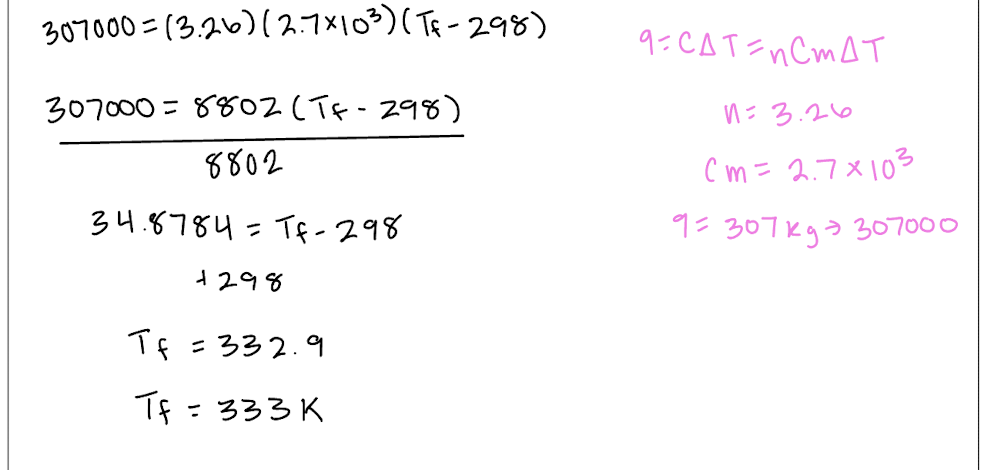
Explain why ΔU=0 for isothermal expansion of an ideal gas.
U is the internal energy of the system, including both kinetic and potential energy. Since this is an ideal gas, no intermolecular forces and so no potential energy, only kinetic. {Ek} is proportional to T and so no change in T means no change in {Ek}. so no change in U