VSEPR Shapes
0.0(0)
Card Sorting
1/13
Earn XP
Description and Tags
Name; bond angle ... COPY THESE LINKS FOR MORE RESOURCES https://phet.colorado.edu/sims/html/molecule-shapes/latest/molecule-shapes_en.html https://youtu.be/aan0AnKKiwQ?si=KXlExMbuwQYttsPk
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
1
New cards
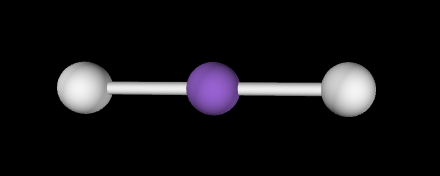
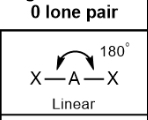
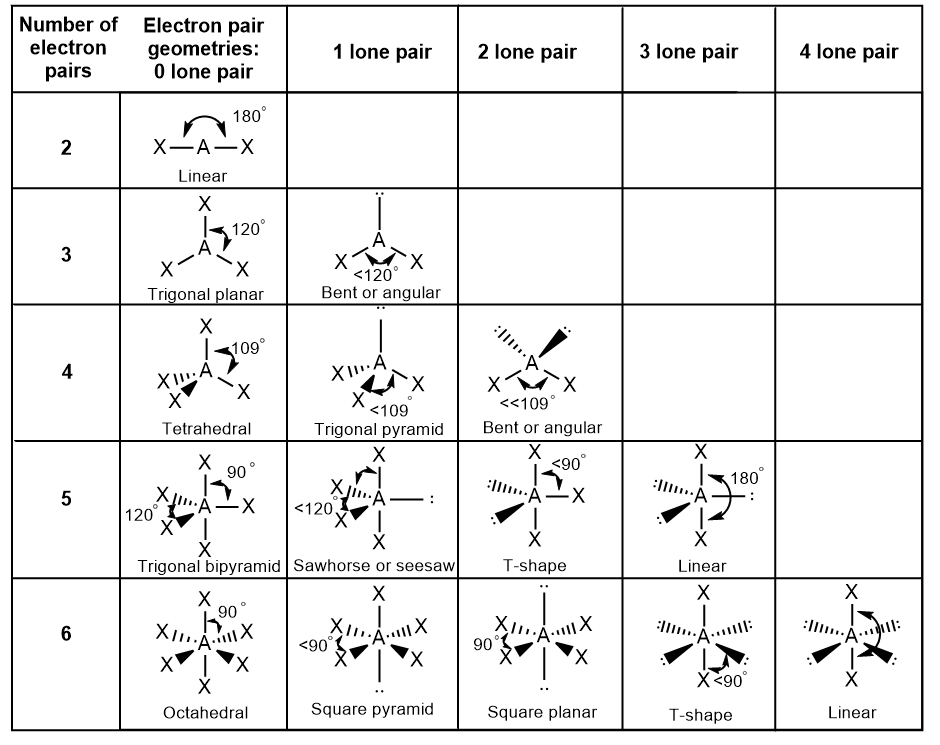
Linear; 180
2
New cards
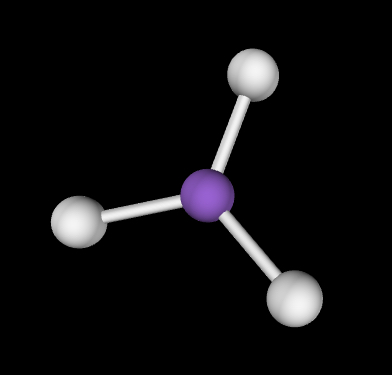
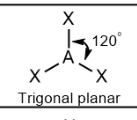
Trigonal planar; 120
3
New cards
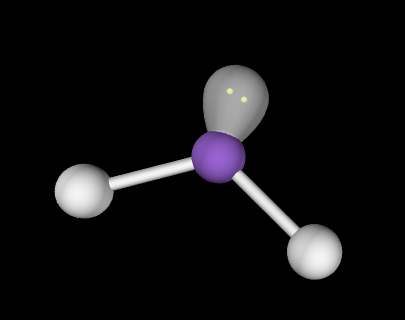
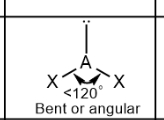
Bent; slightly less than 120
4
New cards
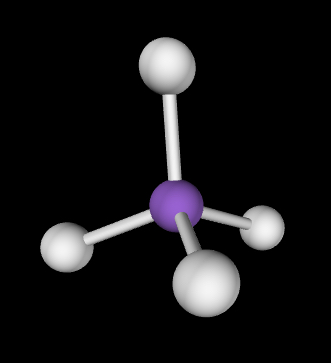
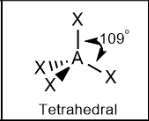
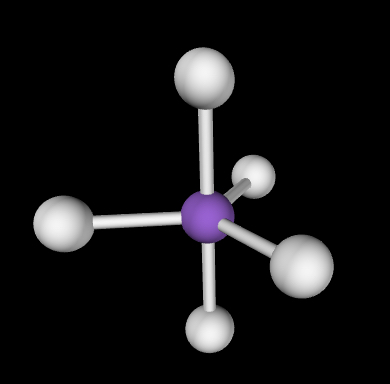
Tetrahedral; 109.5
5
New cards
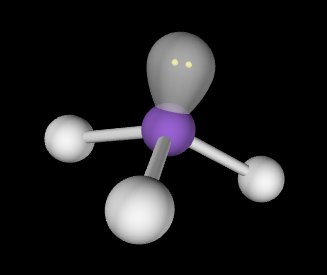
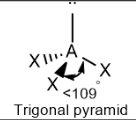
Trigonal pyramidal; slightly less than 109.5
6
New cards
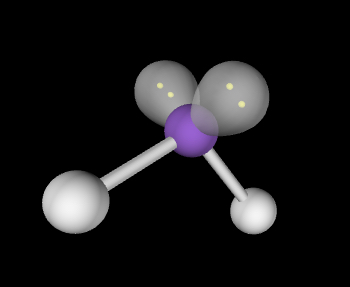
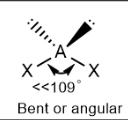
Bent; slightly less than 109.5
7
New cards
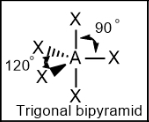
Trigonal bipyramidal; 120 and 90
8
New cards
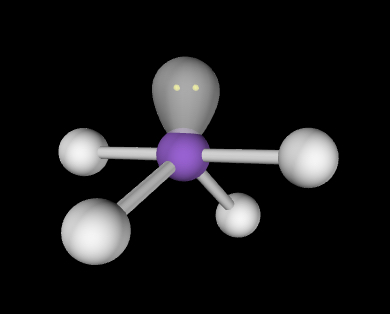
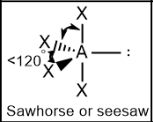
Seesaw; Lone pair = 120; other atoms = slightly less than 90
9
New cards
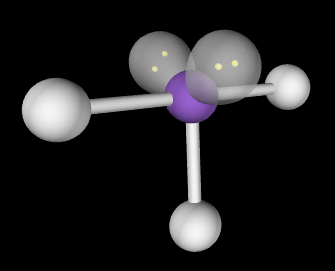
T-shaped; slightly less than 90
10
New cards
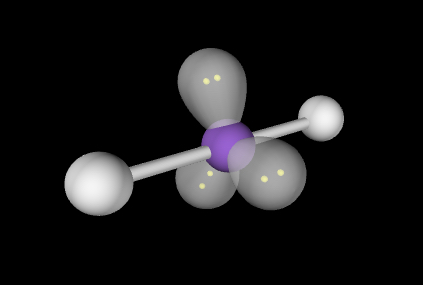
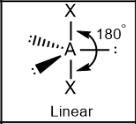
Linear; Lone pairs = 120; other atoms = 180
11
New cards
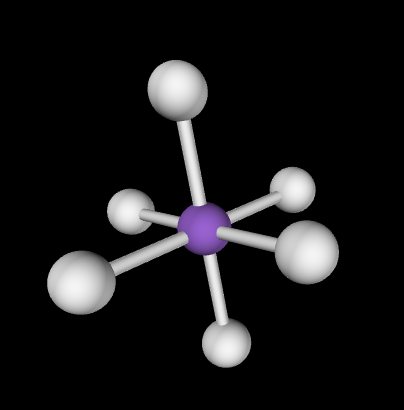
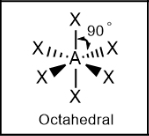
Octahedral; 90
12
New cards
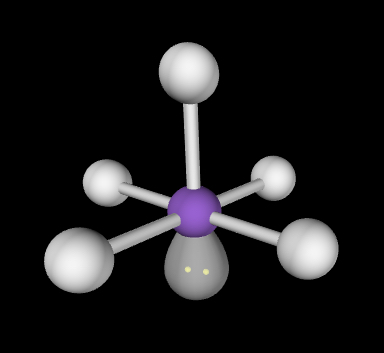
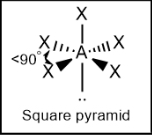
Square pyramidal; between top atom and any base atoms = slightly less than 90
13
New cards
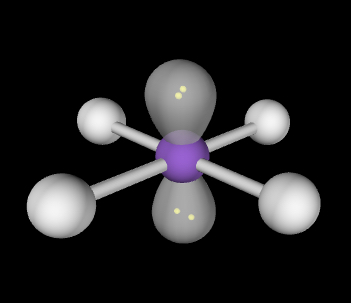
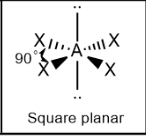
Square planar; 90
14
New cards
me when Valence-Shell Electron-Pair Repulsion