The rate and extent of chemical change
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
What is defined as ‘how quickly a reactant is used up or a product is formed’?
Rate of reaction
Which theory states that for a chemical reaction to happen, reactant particles must collide and have enough energy to react?
Collision theory
What do a the name given to a collision that produces a reaction?
A successful collision
What is defined as ‘the maximum amount of energy needed for a successful collision’?
Activation energy
If the concentration or pressure of a reacting solution is increased, the number of ___ ___ will increase and therefore the ___ ___ ___ will increase
Successful collision, rate of reaction
Large lumps have a (larger/smaller) surface area to volume ratio than smaller lumps or powders
Smaller
If the surface area to volume ratio of a reacting solid is increased, the number of ___ ___ will increase because more reactant particles are ___ at the surface
Successful collisions, exposed
If the temperature of a reaction mixture is increased, the ___ of the particles increases so they move quicker and react with more ___
Energy, force
Does a catalyst alter the products of a reaction?
No
Is a catalyst chemically changed or used up at the end of a reaction?
No
What is the name for catalysts in biological reaction?
Enzymes
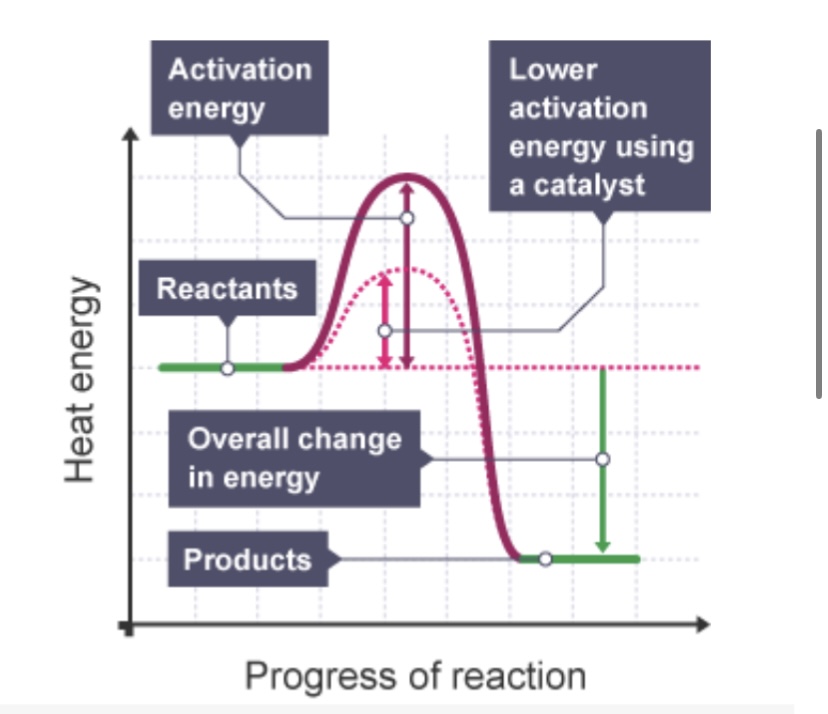
Catalysts provide an alternative activation pathway that has a ___ activation energy than the uncatalysed reaction
Lower
Catalysts (do/don’t) change the frequency of collisions but they (do/don’t) increase the frequency of successful collisions
Don’t, do
When a catalyst is used in a reaction, more particles have energy ___ than the activation energy
Greater
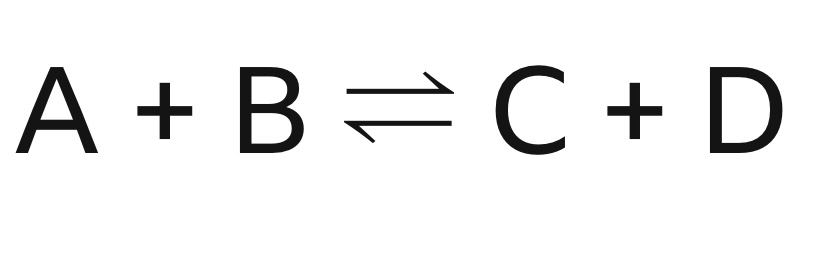
What type of reaction does the image show?
A reversible reaction
In a dynamic equilibrium, the forward and backward reactions have the ___ rate of reaction
Same
The ___ ___ of a reversible reaction is a measure of the concentrations of the reacting substances at equilibrium
Equilibrium position
When a change is made to a system at equilibrium, the position of equilibrium moves to ___ that change
Counteract
If the temperature is increased in reversible reaction, the position of equilibrium moves in the ___ direction to reduce the temperature
Endothermic
If the pressure is increased in a reaction involving gases, the equilibrium position moves in the direction of the ___ moles of gas
Fewest
If the concentration of a reactant is increased, the equilibrium position moves in the direction ___ from this reactant so ___ products are made
Away, more
If a product is removed from a reaction, the position of equilibrium moves to make ___ of that product
More