Science Olympiad Forensics
1/135
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
136 Terms
Sodium flame
yellow flame, very distinct. Even a small amount of sodium will contaminate other compounds.

Lithium flame
carmine or red flame

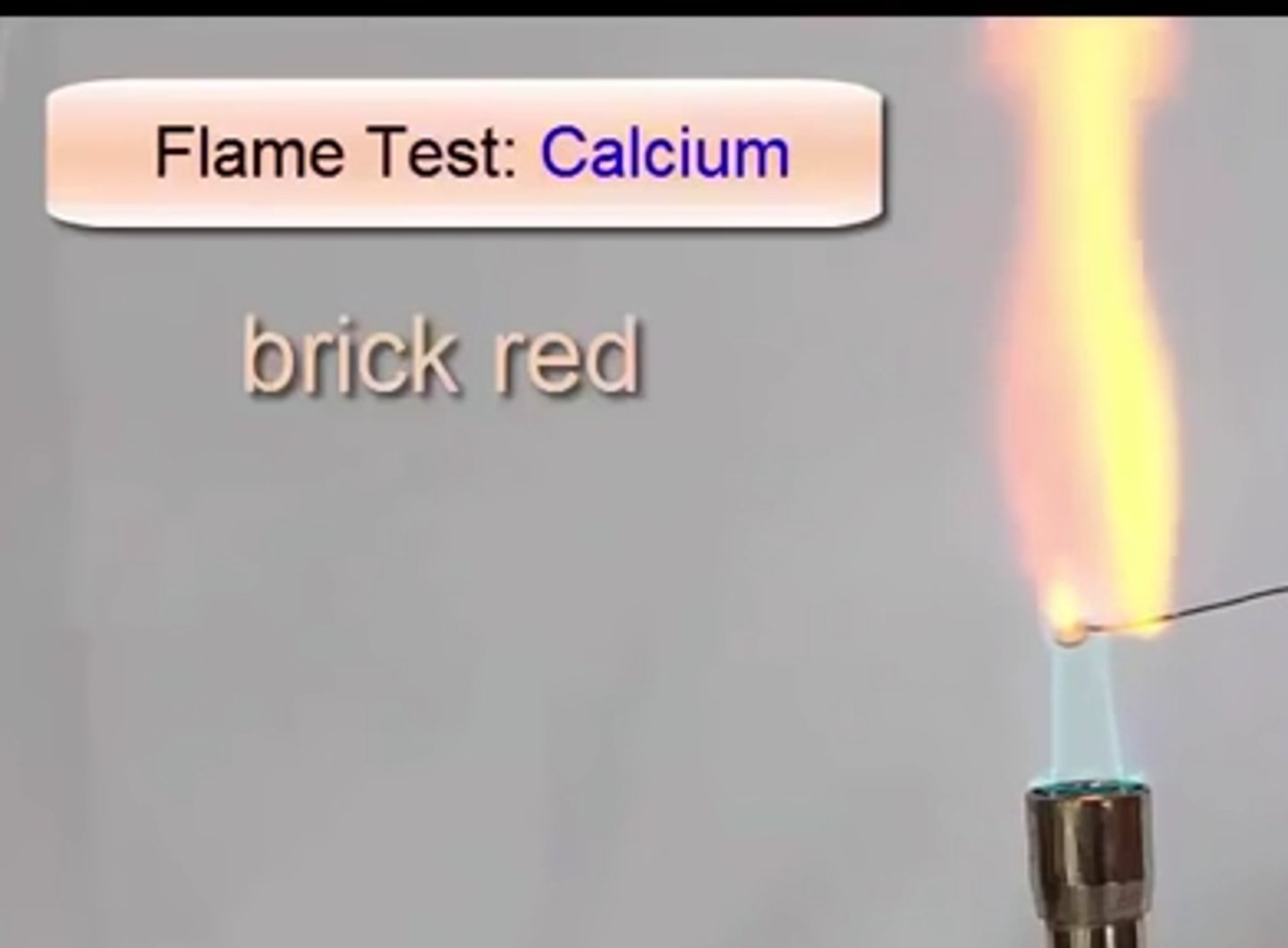
Calcium flame
yellow-red flame
Boric Acid flame
bright green flame, very visible
Ammonium Chloride flame
faint green flame
Potassium flame
light purple, lavender flame

Tests with liquids
Liquids used for identification are iodine, sodium hydroxide, hydrochloric acid, Benedict's solution, and water. Not all liquids are applicable to all samples.
Iodine
When iodine is added to cornstarch, the sample will turn black. If cornstarch is not present, the iodine will remain brown.
Sodium Hydroxide
Sodium hydroxide is used simply to categorize your samples into two fields: NaOH reactive- and non-reactive. For this reason, it is extremely useful when using a flowchart. To perform this test, a few drops of NaOH is added to a small sample of chemical dissolved in water. If a milky-white precipitate forms, the sample is NaOH reactive. If a precipitate does not form, the sample is NaOH non-reactive.
Hydrochloric Acid
Hydrochloric acid will react when added to samples contaning carbonates--therefore, it is useful in identifying calcium carbonate, sodium carbonate, and sodium hydrogen carbonate.
Benedict's solution
Benedict's solution is used to detect glucose. To perform this test, dissolve a small sample of chemical in water in a test tube. Add two to three drops of Benedict's solution, then place the test tube in a hot water bath. If the glucose is present, the sample will react and form an orange precipitate. This test may take a few minutes; be patient. An important fact to note is that sucrose will not react with Benedict's solution but glucose will. Benedict's solution can also be used to test for ammonium chloride. Adding a couple drops will turn the sample a dark blue.
Water
Water is used for determining the solubility of chemical samples, and is used for making solutions.
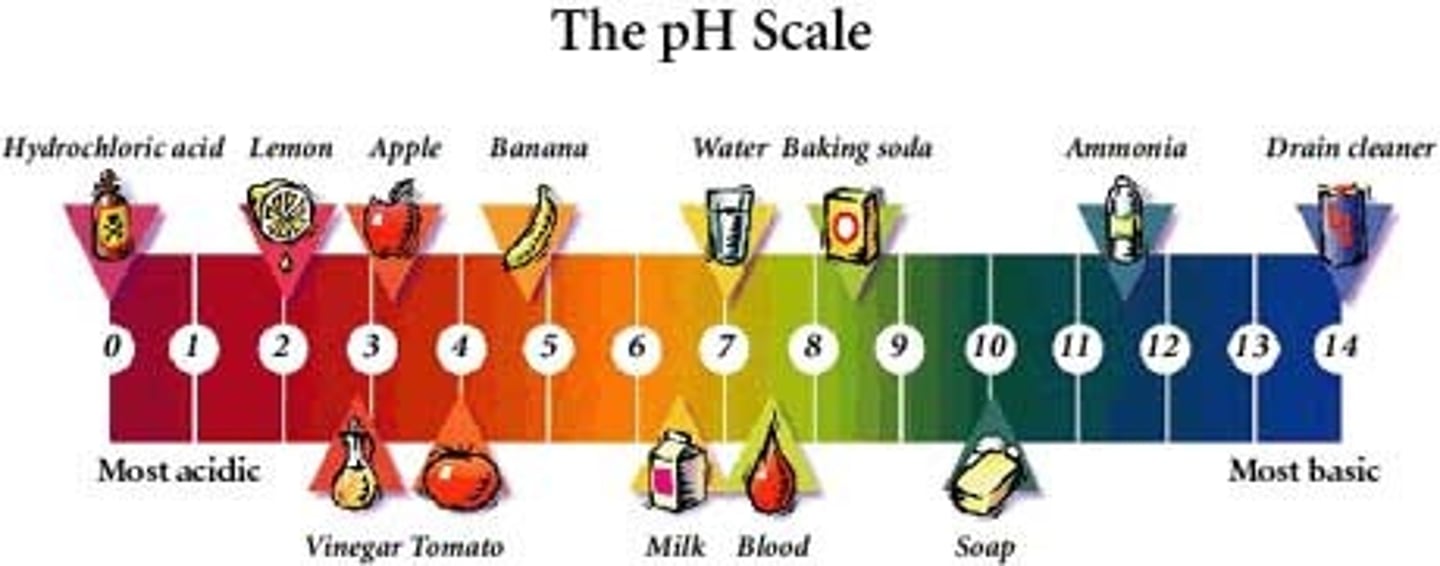
pH
The pH data for chemicals can be useful, especially for determining between two similar chemicals. Most samples have a pH of between 5 and 7, but there are several chemicals that have distinct pH's.
Conductivity
Certain chemical samples will dissociate and become conductive when dissolved in water. To perform this test, dissolve a small sample of dry chemical in water. Using a 9-volt conductivity tester will determine whether a sample is conductive or semi-conductive. This data is especially helpful when following a flowchart.
Solubility
All samples can be divided into two fields--soluble and non-soluble. Water is used to perform this test.
Soluble Samples
sodium acetate, sodium chloride, sodium hydrogen carbonate, sodium carbonate, lithium chloride, potassium chloride, calcium nitrate, glucose, sucrose, magnesium sulfate, boric acid, ammonium chloride
Non-soluble Samples
calcium sulfate, calcium carbonate, cornstarch
Polystyrene (PS)
Yellow flame
• Burns quickly
• Plastic drips
• Illuminating gas odor (naphtha)
• Dense black smoke w/ soot (floating particles)
• CD / DVD jewel cases
• Audio and videocassette casings
• Model assembly kits
• Clear disposable cups
• Styrofoam packaging such as boxes, filler material, etc (EPS)
• Styrofoam tableware such as cups, plates, containers, etc (EPS)
• Building insulation (EPS)
• Cases for electronic equipment such as television, air conditioner, and computer cases (HIPS)
• Stationary such as pen cases, organizing trays, etc (HIPS)
• Toys (HIPS)

Polypropylene (PP)
• Blue, yellow tipped flame
• Burns slowly
• Plastic drips
• Has sweet odor
• Floats in water
• A common use of PP is in food containers. PP is naturally BPA free and has a high melting point making it dishwasher and microwave safe.
• Due to its resistance to fatigue, most hinge type products are also made from PP (such as flip-top bottles, lock&lock Tupperware, etc…).
• PP is often used for storage containers such as Rubbermaid and Sterilite containers. The softer, rubbery lids are made of a softer plastic, usually LDPE.
• Products made from PVC and HDPE can also be made with polypropylene. For instance, the infamous PVC piping can also be manufactured using PP. HDPE furniture such as tables and chairs can also be substituted using PP.
• PP is commonly used in non-woven fabrics (used in diapers and or sanitary products).
• Polypropylene is commonly used for producing ropes, carpets and recycled plastic rugs.
• Many stationary products such as plastic folders, notebook covers, paper protectors, storage boxes are also made from PP. These products are made through the plastic extrusion process.
Polyvinyl Chloride (PVC)
• Yellow flame w/ green spurts
• Plastic does not drip
• Self extinguishing
• Smells like hydrochloric acid
• Plastic chars
• A large usage of flexible PVC is in wire insulation (colored plastic wrapped around electrical wires). Flexible PVC can be found in clothing such as raincoats, rain boots, and leather-like fabrics. PVCs are also made into vinyl records and vinyl signs and billboards.
• About 75% of all PVC resin (rigid) is made into construction materials such as piping & fittings, siding, flooring, windows, fencing, decking, roofing, wall coverings, etc

Low Density Polyethylene (LDPE)
food containers (specifically bags), grocery bags, plastic wrap, etc.

High Density Polyethylene (HDPE)
food containers, bags, lumber, furniture, flower pots, signs, trash cans, toys

Polycarbonate (PC)
• Orange flame
• Self extinguishing
• Plastic drips
• Black smoke w/ soot (floating particles)
• Faint, sweet aromatic odor
• Data storage including, CDs, DVDs, blu-ray discs, etc…
• Lenses including sunglasses, prescription glasses, automotive headlamps, riot shields, instrument panels, etc
• PC is derived from bisphenol A (BPA) and is no longer used in food applications
• Electrical and telecommunications hardware
• Construction materials such as dome lights, sound walls, etc
• Automotive, aircraft, and security components
• Medical applications

Polyethylene Terephthalate (PET or PETE)
• Yellow flame
• Plastic drips
• Burns slowly
• light smoke
• Polyethylene Terephthalate is probably most well known for its use in water, juice, and soda bottles. You’ll also find PET plastic used in other packaging such as peanut butter jars, containers for holding salad dressings, cooking oils, cosmetics, and household cleaners. PET used for plastic packaging consumes roughly 30% of PET usage worldwide.
• A major use of Polyethylene Terephthalate is in synthetic fibers used for manufacturing polyester clothing, fabrics, carpets, etc. PET used for this purpose consumes more than 60% of PET usage worldwide!

Polymethyl Methacrylate (PMMA)
Plexiglas, glass substitute
Wool
- Animal
- Most commonly used animal fiber
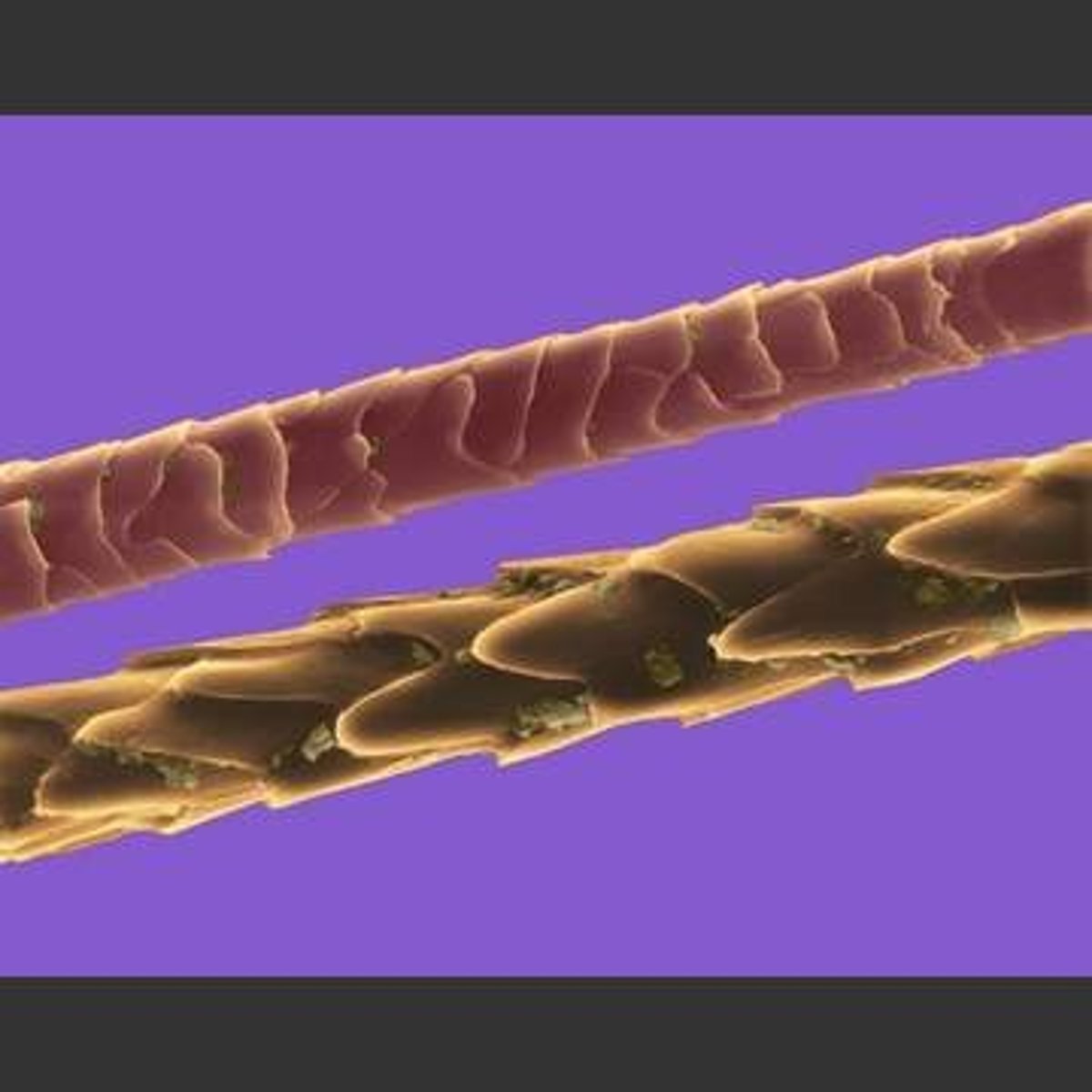
- shrivels, leaves brown-black residue, smells like burning hair
- cylinder with scales
Silk
- Animal
- Smoother than wool
- shrivels, leaves black residue, smells like burning hair
- thin, long and smooth cylinder
Cotton
- Vegetable
- Most widely used plant fiber, fairly short fibers
- burns with a steady flame, smells like burning paper, able to blow flame from thread like a match, leaves a charred whitish ash
- irregular twisted ribbon
Linen
-Vegetable
- fibers generally longer and smoother than cotton
- burns at a constant rate, does not produce smoke, smells like burning grass, produces sparks
- smooth, bamboo like structure
Polyester
- Synthetic
- fibers can be any length
- melts, only ignites when in the flame, drips when it burns and bonds quickly to any surface it drips on, produces sweet odor and hard, colored (same as fiber) ash
- completely smooth cylinder
Nylon
- Synthetic
- long fibers
- curls, melts, produces black residue, smells like burning plastic (some sources say it smells like celery?), ignites only when brought into flame
- fine, round, smooth, translucent
Spandex
- Synthetic
- can stretch to eight times its original length
- melts quickly
- Flattened, ridged fibers, clustered
Human Hair
- scaly cuticle (called imbricate)
- amorphous medulla, very thin if visible at all, can possess fragmented or absent medulla
- cuticle pattern resembles that of unorganized, overlapping roof shingles.

Cat Hair
- usually finer than dog or human hair
- medulla is thick, discontinuous or ladder pattern
spinous-scaled cuticle very visible - looks like palm tree
Dog Hair
- can be coarse or fine, depending on location hair is from
medulla is continuous, vacuolated to amorphous,
- occasionally very broad
- scales of cuticle not very visible

Horse Hair
- very coarse, thick
- medulla is absent to unbroken; cellular or amorphous (mosaic pattern)
- imbricate scales on cuticle

Bat Hair
- very fine
- distinguishable by coronal scales on cuticle - looks like a stack of paper cups

Chromatography
There are two types of chromatography, which are paper chromatography and TLC (thin layer chromatography).
RF
retention factor or rate of flow.
Mass Spectrometry
an analytical method used to determine the mass to charge ratio of charged particles.
FIngerprints
Fingerprints are formed by the arrangement of volmer (or volmar) pads. They are made mostly of sweat and water but can also contain various organic and nonorganic compounds.
Plain Whorl

Ulnar Loop

Radial Loop

Plain Arch

Tented Arch

Central Pocket Loop

Double Loop

Accidental Whorl

Visible Print
As the name suggests, these ones can easily be seen because they were made with a substance like ink or blood. They can also easily be photographed without development.
Impression
Made in soft material such as clay. Less easy to detect than visible fingerprints, but can still be photographed without development.
Latent
Invisible fingerprints. These must be developed before photographed.
Dusting
Powder applied to prints sticks to fatty acids and lipids.
Iodine Fuming
an excellent way to develop prints on flexible, porous and non-porous surfaces such as paper, index cards, magazines, and cardboard. To fume a suspected latent print, the surface must be placed into a container with solid iodine. The sublimation of iodine in a closed container will cause iodine vapors to concentrate, then be absorbed by the oil and sweat left behind by human skin. The temporarily-developed print will then be visible as an orange/brown outline. Upon development, the print should be photographed for documentation. The iodine will eventually sublime from the surface of the print, allowing the print to return to its latent state. The surface is then returned to its original appearance and can even be exposed to additional developing techniques.
Ninhydrin
Ninhydrin is a chemical that reacts with amino acids to form a purple compound. This development technique is used primarily on porous surfaces such as paper, tissue, and clothing. The white powder ninhydrin must be dissolved in acetone before it can be soaked into a surface for development. The reaction will then develop the print within twenty four hours, though that time can be significantly reduced if heated the treated print is heated during development.
Cyanoacrylate (Superglue) Fuming
also called superglue method. Most liquid super glues are really either methylcyanoacrylate or ethylcyanoacrylate. Less common types of super glue include butylcyanoacrylate and isobutylcyanoacrylate. Fortunately, all these types of super glue are nearly identical physically and chemically. Super glue reacts with the traces of amino acids, fatty acids, and proteins in the latent fingerprint and the moisture in the air to produce a visible, sticky white material that forms along the ridges of the fingerprint. The final result is an image of the entire latent fingerprint. This image can be photographed directly, or after further enhancement. However, the glue must be in gaseous state. To do this, one places the surface suspected of containing a latent fingerprint in a container with a heater in it. Then, they place a small, opened container of the glue on top of the heater, and carefully seal the overall one. After that, simply wait.
Small Particle Reagent (SPR)
Not as common as the other methods used, but still important. SPR is used for wet surfaces and reacts with the lipids present in fingerprints.
DNA
deoxyribonucleic acid
four nucleotides
adenine, cytosine, thymine, and guanine
gel electrophoresis
When the current runs through the gel during this process, because DNA is negatively charged, it will move towards the positive end of the box. Smaller fragments of DNA will obviously move farther through the gel filter than larger ones.
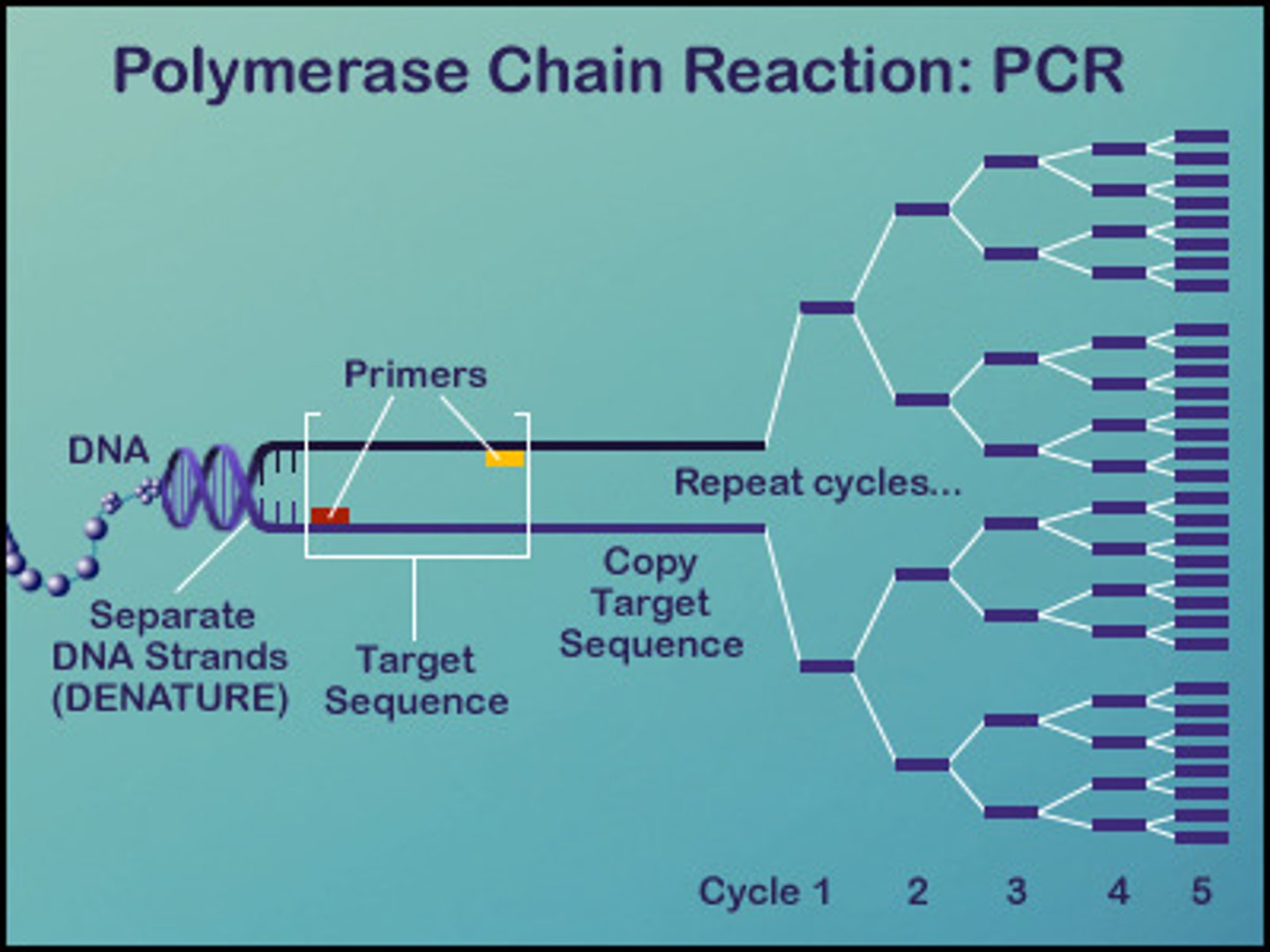
PCR Polymerase Chain Reaction
synthetic DNA replication developed in 20th century
Glass Fractures
- Cracks end at existing cracks
- A small force forms circular cracks
- Radial cracks and chonchoidal cracks make right angles, but face different ways. When dealing with fractures, remember the 3Rs of glass fracture: Radial cracks at Right angles on the Reverse side of impact.
- A force very close to the glass before impact, such as a gunshot or a rock, will completely shatter the glass
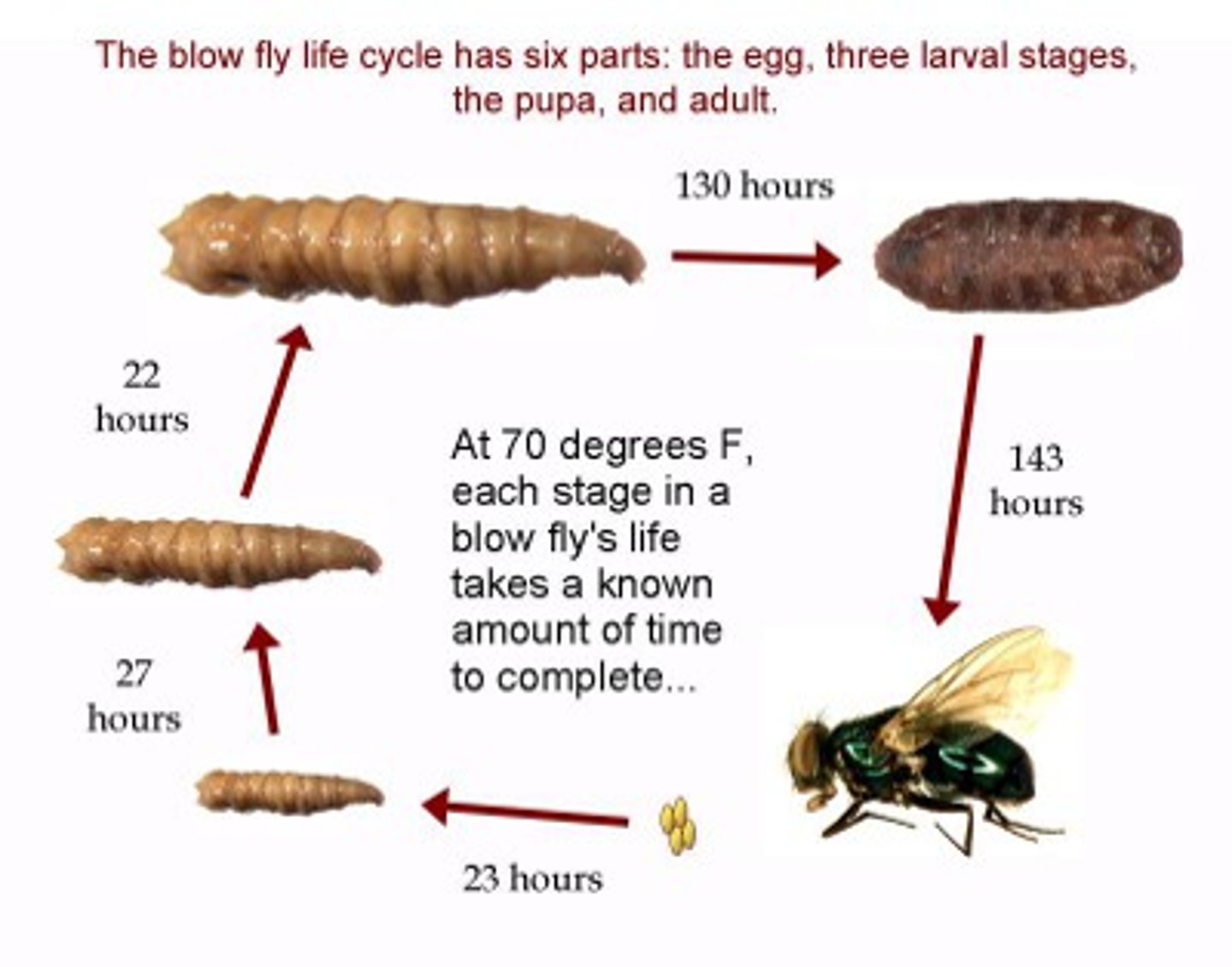
Entomology
Stages of insects found on a dead body can tell how long the victim has been dead.
Blood Spatters
generally classified by velocity at which they form
Low Velocity Formation Blood Splatter
A low-velocity spatter is usually the result of dripping blood. The force of impact is five feet per second or less, and the size of the droplets is somewhere between four and eight millimeters (0.16 to 0.31 inches). This type of blood spatter often occurs after a victim initially sustains an injury, not during the infliction of the injury itself. For example, if the victim is stabbed and then walks around bleeding, the resulting drops are a type of low-velocity spatters known as passive spatters. Low-velocity spatters can also result from pools of blood around the body of a victim and transfers (impressions left by weapons, or smears and trails left by movement). It can occur with some injuries, such as bleeding sustained from a punch.
Medium Velocity Formation Blood Splatter
A medium-velocity spatter is one that has a force of anywhere from 5 to 100 feet per second, and its diameter is one to four millimeters.
It can be caused by a blunt object, such as a bat or an intense beating with a fist. It can also result from a stabbing. Unlike with a low-density spatter, when a victim is beaten or stabbed, arteries can be damaged. If they're close to the skin, the victim bleeds faster and blood can spurt from wounds as his or her heart continues to pump. This results in a larger amount of blood and a very distinctive pattern. Remember that if a victim is beaten, there is usually an initial blow which does not result in blood spatter since there is no exposed blood. Also, medium velocity blood spatters can result from blood being flung from a weapon that has already hit the victim once, and is now being going in for another hit. This forms a distinctive pattern on the wall which shows the exact movement of said weapon
High Velocity Formation Blood Splatter
Classified by blood drops traveling through the air at over a hundred feet per second, this can appear as either a mist, or a fine spray of droplets between .1 and 1 millimeter in diameter (these can help determine the direction from which the victim was hit).
Angle of Impact
The angle at which a spatter hits a surface. The formula for it is:
θ=arcsin(W/L)
Where theta (θ) is the angle, W is the width of the spatter, and L is the length.
Note that arcsin is also known as inverse sine.
Tracks
evidence of footprints or tire tracks, checking for pattern. shape, or form
Soil
connect a suspect to the general area of the crime
Blood Types
A, B, AB, O

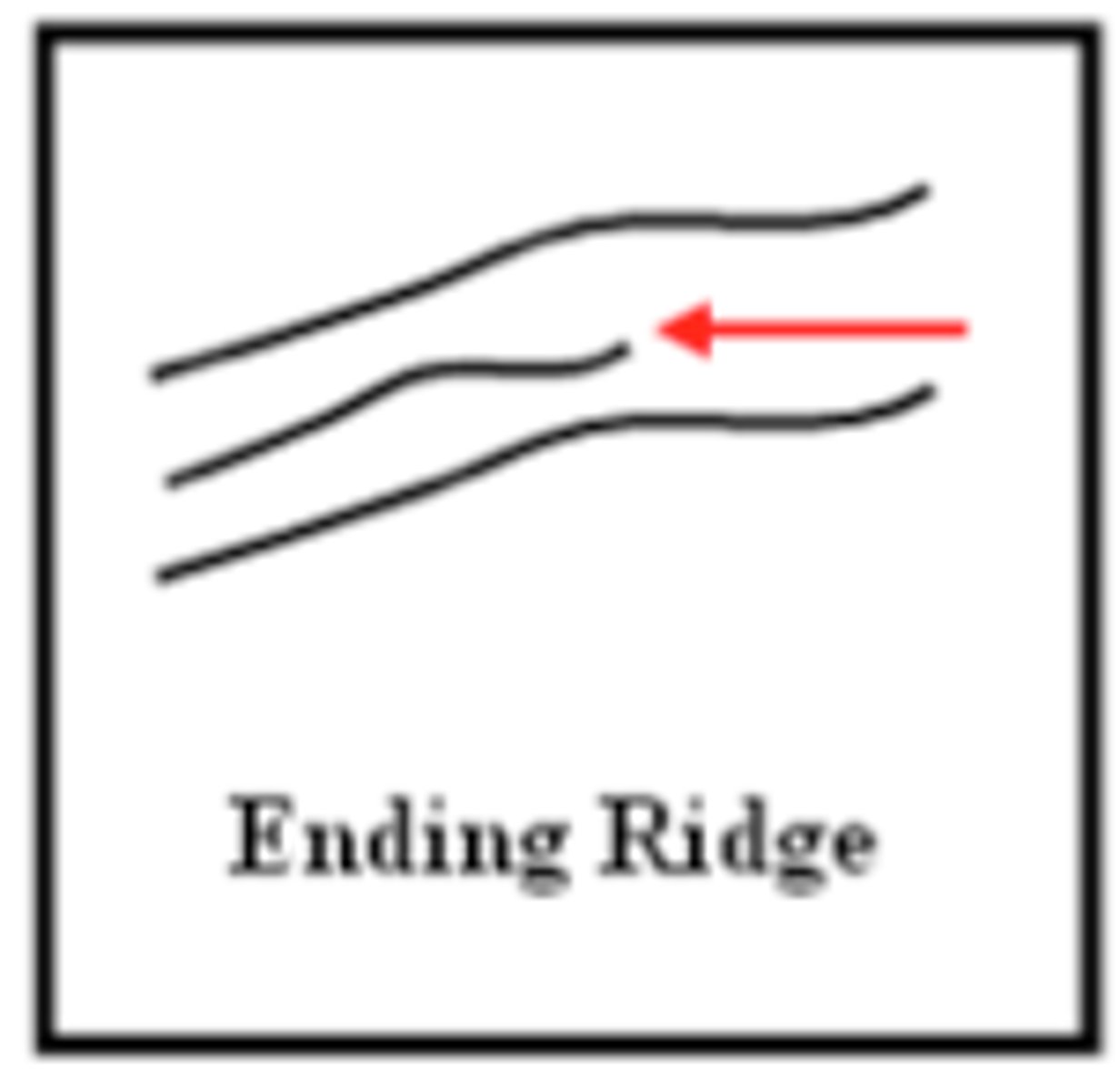
ridge ending
the line-like, raised formations that form the pattern that forensic scientists use to analyze and identify fingerprints. By all means, they alone with the furrows they create define the fingerprints. When they form is when a fetus gets his/her fingerprints (more notes on fingerprint formation are listed below)
bifurcation
a point on a fingerprint in which a single ridge divides into two

dot
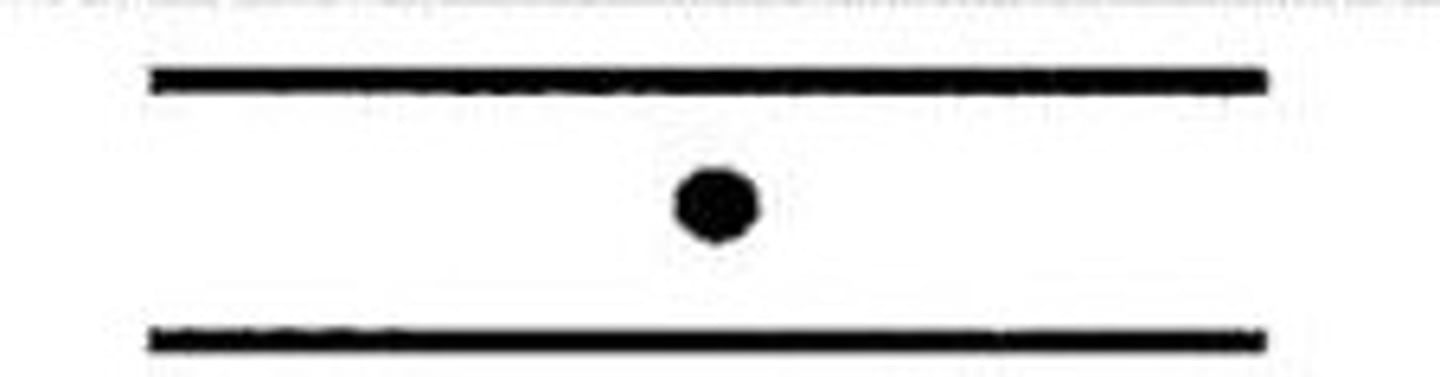
island (short ridge)
formation in which the ridge is shaped like a dot
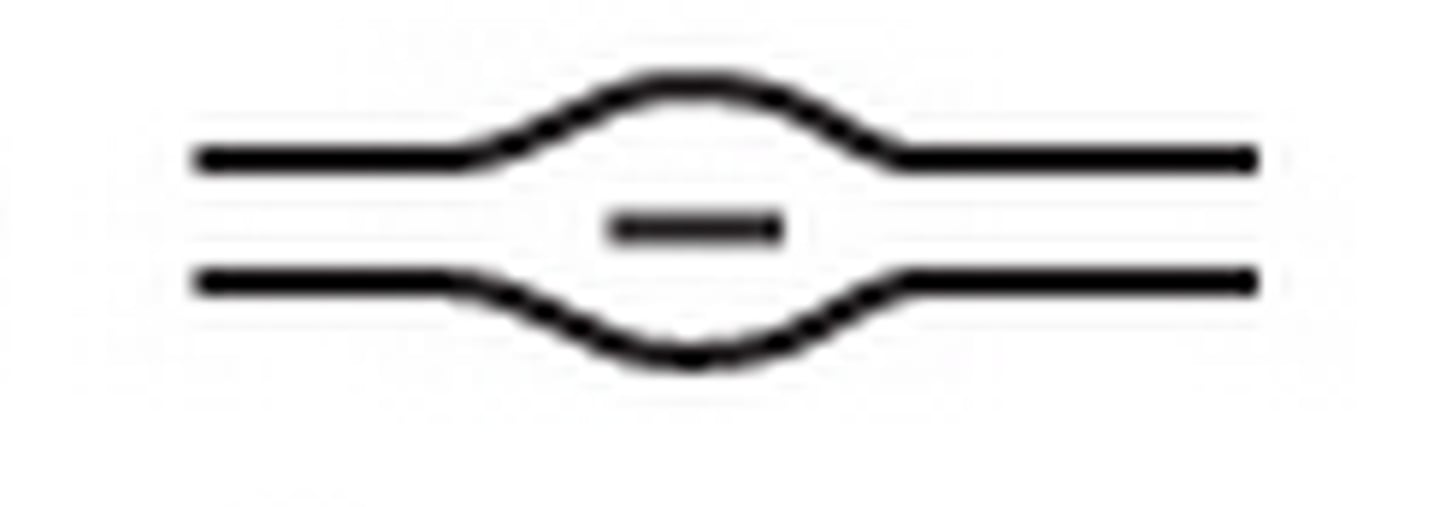
lake (enclosure)
an often elliptically-shaped, bowl-like furrow surrounded by ridges

hook (spur)
one line branching off from the others

bridge
a bridge between one fingerprint ridge and another
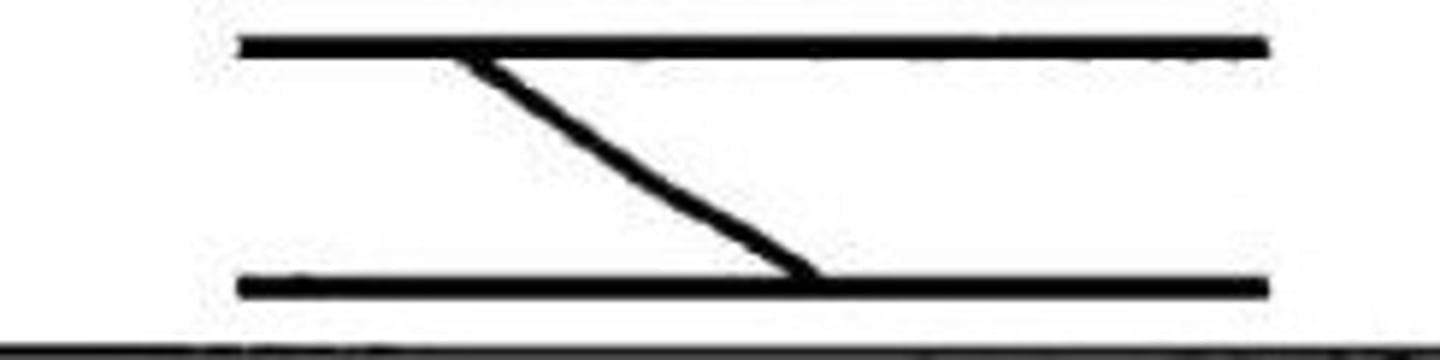
double bifurcation
Occurs when a ridge splits or forks into two separate ridges and the splits again into two separate rides

trifurication
Splitting into three branches

opposed bifurcations
opposite "v" shapes on the same line

ridge crossing
two ridges merge then immediately split

opposed bifurcation (ridge ending)
a "v" shape ending early

Luminol (C8H7N3O2)
a white to pale yellow crystalline solid that is solid in most polar organic solvents, insoluble in water
medulla
thin, central structure of the follicle whose function is undetermined. In forensics, it is important to remember that not all hair follicles found may be human. To tell the species of origin, forensic analysts look to see the pattern of fractures on the medulla, if any.
cortex
the middle layer of the follicle containing the pigments: made up of a complex pattern of air pockets, and differently shaped pigment particles. Forensic analysts take advantage of these unique arrangements to identify whose hair it is
cuticle
the external layer of cells resembling a shell of translucent fish scales or a mosaic. Forensic analysts can use the pattern to determine species of origin and possibly match the follicle to another human if that's what it is
root
the part of the hair located under the skin (all previously mentioned components of the follicle are located in the shaft, which is the part sticking out of the skin) in the tube-like structure known as the follicle. It is where the hair first begins to grow, the underlying cells forming the protein keratin which comprises the hair.
Describe the hair follicle of a caucasian
Shaft diameter: moderate with little variation, Cross Section: oval, Pigment granules evenly distributed
Describe the hair follicle of an African
Shaft diameter: fine to moderate with considerable variation, Cross Section: flattened, Pigment granules: clumped
Describe the hair follicle of an Asian
Shaft diameter: moderate with little variation, Cross Section: round, Pigment granules: large patchy areas
loop
the most common basic pattern of the human fingerprint, formed by several sharply rising elongated-U-shaped ridges
whorl
one of the basic patterns of the human fingerprint, formed by several complete circular ridges one inside another
arch
one of the basic patterns of the human fingerprint, formed by several complete circular ridges one inside another
two types of fingerprint powder
regular and magnetic
regular powder
preferable when dusting off such surfaces as windows, televisions, kitchen counter tops, table tops, painted surfaces, cabinets and many other surfaces found in residential and commercial settings, and it is quite effective in dustings on apprehended or stolen vehicles on painted surfaces on the exterior of the vehicle and on glass. It also has the advantage of being available in different colors ranging from black to pink, which makes the fingerprint copy generally more discernible against any debris that the adhesive tape may peel off the surface as well. When utilizing this type of powder, one should use a fiberglass brush. Also, it is important to not get too much powder on it at a time, or you risk compromising the fingerprint. Lightly dapple the tip.
magnetic powder
Magnetic powders are best applied to shiny surfaces, such as plastic containers. When dusting for fingerprints with magnetic powder, crime scene investigators must use a magnetic applicator which has a magnet. Magnetic powder is applied with a light hand with brushing strokes. Besides being available in the colors of black, white, silver/gray and biochromatic, magnetic powder is also available in fluorescent magnetic powder colors like red and green. These can be used when dealing with problematic background fluorescence, and they are best applied with feather duster.
paper chromatography
How to Perform a Chromatogram on a Pen:
1.Take a wide rectangular piece of filter paper and use a pencil to draw a line
about 1.5 cm from the bottom edge of 1 long side.
2. Place small dots of each of the known materials you are to test spaced about 1
cm apart along the line you just drew. Be sure to label what the dots are on the
paper ABOVE the dot.
3. Roll the paper up like a cylinder & staple the ends together.
4. Put about 1 cm of water in a Petrie dish.
5. Set your chromatography paper in the dish with the dot side down. Be sure the
water level in the Petrie dish does not touch any of the dots on the line you made.
6. While that chromatogram is developing do the same thing over for the knowns
of another set of materials.
7. Take 2 strips of chromatogram paper and make a line about 1.5 cm from the
bottom with a pencil on each.
8. Bring your prepared pieces of chromatogram paper to the instructor who will
place a dot on the papers as your unknowns.
9. Hang the papers in a beaker by placing a wooden stick through the hole in the
top & resting the wooden stick on the lip of the beaker.
10. Use your wash bottle to put enough water in the beaker so that the bottom of
the chromatograph paper just is in the water, but not so much that the dots are in
the water. Be sure that you do not get any water on the chromatography paper as
you are putting it in.
11. The chromatograms are done when the water level gets up to about 1.5 cm
from the top.
12. Take the chromatograms out and spread them on a paper towel to dry.
13. Which of the knowns do your "crime scene" chromatograms match up to?
How do you perfrom DNA electrophoresis?
1. Splice the DNA into fragments via restrictor enzymes.
2. Insert the fragments into the small pits at the top of a electrophoregram gel pad (put all of the fragments from a single DNA sample into one pit)
3. Put the pad into the gel electrophoresis machine.
4. When it is done, look at the pad. If the dark spots created by a DNA sample are in the same place related to the length of the pad as another sample, the two belong to the same person.
What really happens in DNA electrophoresis?
The electrophoresis machine creates a negative electric current at the top of the gel pad (the place where the pits are located) and a positive current at the bottom. This causes the DNA fragments to run down the gel pad. Each fragment is a different size and weight. The heavier it is, the closer to the top it will be, while the lighter, the farther it will run down to the bottom. In the places where a DNA fragment remains, a dark splotch shows up on the pad. If the same type of enzyme is used as the restrictor enzyme, the same distribution should occur in the any sample of the same DNA. That means the splotches for each sample of the same DNA will be in the same place lengthwise along the pad, allowing you to determine if the two samples do in fact belong to the same person.