MCAT General Chemistry - Oxidation–Reduction Reactions
1/20
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
Leigh disease
extremely rare mitochondrial disorder; number of key mitochondrial enzymes are disrupted and the process of oxidative phosphorylation is never achieved; when pyruvate cannot be oxidized to acetyl-CoA, it is instead fermented to lactic acid
Oxidation–Reduction (redox) reactions
Reactions that involve the transfer of electrons from one chemical species to another; loss and gain must happen simultaneously
oxidation
loss of electrons
reduction
gain of electrons
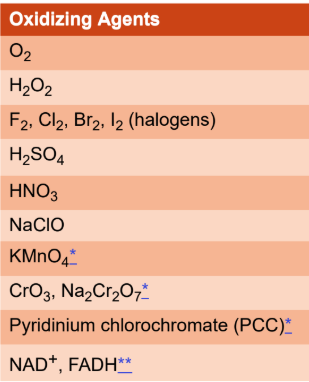
oxidizing agent
causes another atom in a redox reaction to undergo oxidation and is itself reduced; applied specifically to the atom that gains electrons
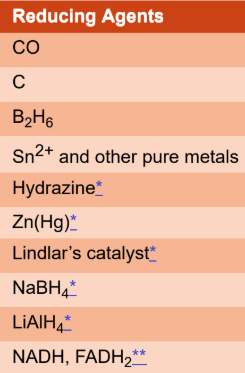
reducing agent
causes the other atom to be reduced and is itself oxidized; applied specifically to the atom that loses electrons
Oxidation numbers
assigned to atoms in order to keep track of the redistribution of electrons during chemical reactions; typical charge of an element based on its group number, metallicity, and general location in the periodic table; assumes unequal division of electrons in bonds, “awarding” the electrons to the more electronegative element
Oxidation Rules
Free element = 0
N2 = 0
He = 0
Monatomic ion = charge
Cu2+ = +2
Fe3+ = +3
N3- = -3
Group IA = +1
Na+ = +1
Group IIA = +2
Ca2+ = +2
Group VIIA = -1 except when combined with an element of higher electronegativity
HCl - Cl- = -1
HOCl - Cl+ = +1
Hydrogen = +1, except with less electronegative elements
HCl - H+ = +1
NaH - H- = -1
Oxygen = -2, except peroxides (-1 each) and more electronegative atoms
OF2 - O2+ = +2
Sum of oxidation numbers = 0 or charge of ion
formal charge
not the same as oxidation number; assumes equal division of electrons in bonds, “awarding” one electron to each atom in the bond
half-reaction / ion–electron method
equation is separated into two half-reactions—the oxidation part and the reduction part; Each half-reaction is balanced separately, and then added to give a balanced overall reaction
complete ionic equation
split the various species into all of the ions present
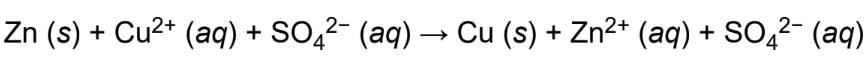
spectator ions
chemically inert ion during a reaction; not taking part in the overall reaction but simply remaining in the solution unchanged
net ionic equation
showing only the species that actually participate in the reaction
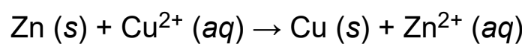
combination reactions
two or more species come together to form a product
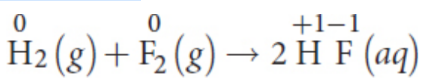
decomposition reactions
one product breaks down into two or more species
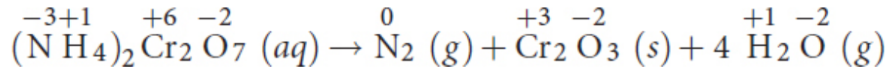
combustion reactions
fuel (usually a hydrocarbon) is mixed with an oxidant (usually oxygen), forming carbon dioxide and water
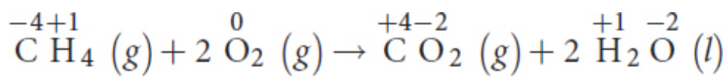
Double-displacement / metathesis reactions
involve the switching of counterions; ions generally retain their oxidation state, so not usually Oxidation–Reduction reactions
Disproportionation (dismutation)
specific type of redox reaction in which an element undergoes both oxidation and reduction in producing its products
ex. many biological enzymes (catalase, superoxide dismutase)
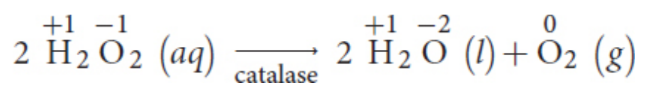
Redox titrations
follow the transfer of charge (as electrons) to reach the equivalence point; utilize indicators that change color at a particular voltage (emf) value
iodimetric titration
use of starch indicators to identify iodine complexes; use of starch indicators to identify iodine complexes; dark solution in the presence of starch, and at the endpoint of the titration, a colorless solution develops
Potentiometric titration
redox titration where no indicator is used; electrical potential difference (voltage) is measured using a voltmeter