9.7 ocean acidification + questions
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
31 Terms
Which of the following causes ocean warming?
Greenhouse gases in the atmosphere.
As ocean temperatures are warming globally, many marine species are being affected. Which of the following are expected effects?
Metabolic and reproductive changes
Which of the following are ALL effects of ocean warming on marine species?
Loss of habitat, increase in invasive species, and damage to coral skeletons
Which of the following best describes coral bleaching?
Coral bleaching results from the loss of algae within corals as ocean temperatures rise
Which of the following best describes how greenhouse gasses cause ocean warming?
Greenhouse gasses cause an increase in global temperature overall, which is ambiently transferred to oceans, raising the water temperature
Which of the following explains how ocean acidification decreases populations of aquatic organisms such as oysters, urchins, and coral?
By increasing hydrogen ions in the water, there are fewer carbonate ions available for organisms to build calcium carbonate-based structures, such as shells or coral skeletons
Which of the following results from increased atmospheric CO2 leading to an increase in dissolved CO2 in the oceans?
The decrease in ocean pH levels
what determines the PH of a solution
hydrogen ions
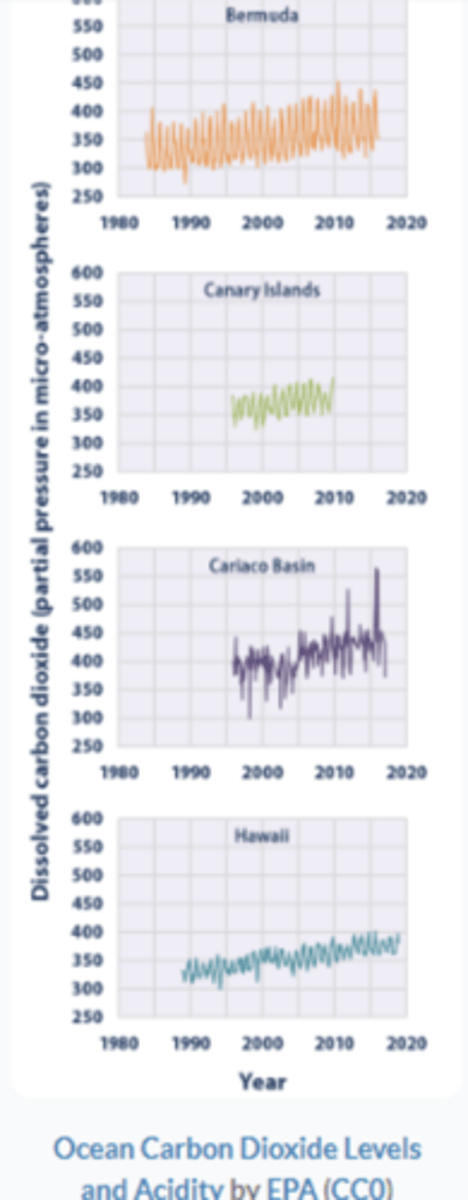
According to the trends seen in the carbon dioxide data in the graph to the right, which of the locations is likely experiencing the most acidification?
Cariaco Basin
A researcher living in a major port city noticed an increase in shipping traffic by boat in the local waters. In order to determine whether the shipping industry was impacting the conditions of the ocean, they designed an experiment to test the acidity of the ocean water over time. The researcher gathered data on the number of ships present and the pH level of the water over the course of two years. These measurements were taken as a monthly average, and the data confirmed that pH dropped slightly as boat traffic increased.
Which of the following was the independent variable in this experiment?
The amount of boat traffic
Which of the following explains how oceans are affected by increased CO2 concentrations in the atmosphere?
Increased CO2 concentrations in the atmosphere would decrease pH levels in the ocean
how does ocean acidification start?
to much CO2 (carbon dioxide) in the atmosphere
what does to much CO2 in the atmosphere mean
the oceans are absorbing alot of that CO2
why do we have excess CO2 in the atmosphere
because we are burning fossil fuels therefore creating more CO2
what does burning of fossil fuels create
electricity
what causes global warming
the trapping of heat from the excess amount of CO2 (some of it gets absorbed by oceans the rest gets trapped as a green house gas)
when the water absorbs the
carbonic acid (H2CO3) (step 1)
CO2 + H2O =
H2CO3 (carbonic acid)
carbonic acid
is basically sodas
step 2
H2CO3 dissociates (breaks down) into bicarbonate, carbonate and H+ ions
bicarbonate
H-3
carbonate
CO-23
when carbonate ions free floating in the water they can combine with
free floating hydrogen ions to form more bicarbonate
when you measure PH you are basically measuring
the concentration of hydrogen ions
the more hydrogen ions we have in a solution
the more acidic it is
what changes the Ph of the water
the free floating hydrogen ions
how does calcium carbonate and marine life get affected by ocean acidification
shells break down as PH decreases (gets weaker) because their is not enough carbonate for them to strengthen their shells
ocean acidification makes carbonate ions
less available
what dont organisms or shelled organisms not want to use
bicarbonate, they want to use carbonate instead
any organisms that has a shell or exoskeleton is finding it more difficult to make their shell because
their is fewer carbonate ions in the water (because carbonate ions are bonding to hydrogen ions to form bicarbonate)(big impact)
how are humans causing ocean acidification
-combustion of fossil fuels
-deforestation (more CO2 in atmosphere)
-combustion of coal (leads to acid rain which will decrease the ph of the ocean which makes it more acidic)