Alcohols, Phenols, and Ethers in Chemistry
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
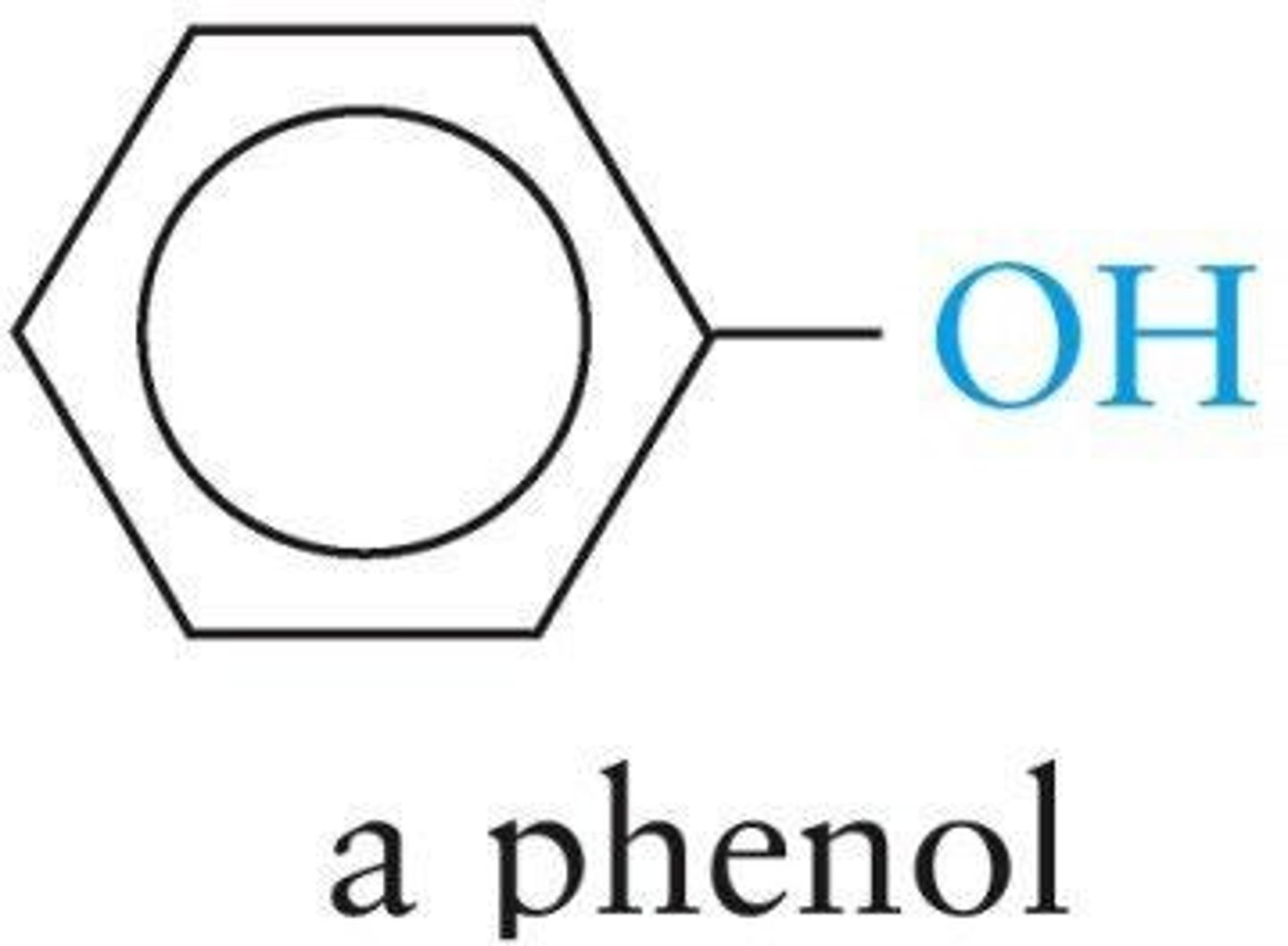
Phenol
Compound in which an —OH group is connected to a benzene ring
Diols
Alcohols containing two —OH groups
Triols
Alcohols containing three —OH groups
Solubility of Alcohols with three carbon atoms or fewer
Infinitely soluble in water
Solubility of Alcohols with four or more carbon atoms
Have limited solubility in water
Effect of added -OH groups on solubility
Increase the solubility
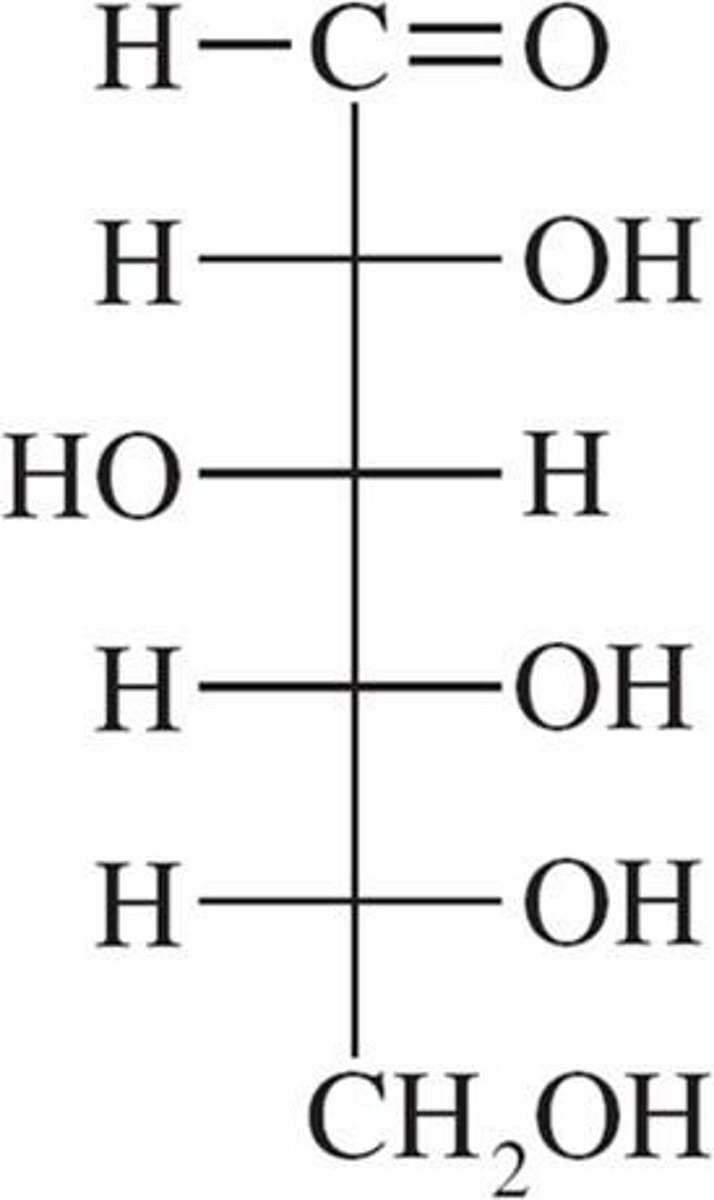
D-glucose
Solubility = 95g/100g H2O
Hydrogen bonds in alcohols
The —OH group can make hydrogen bonds between alcohol molecules that lead to relatively high boiling points.
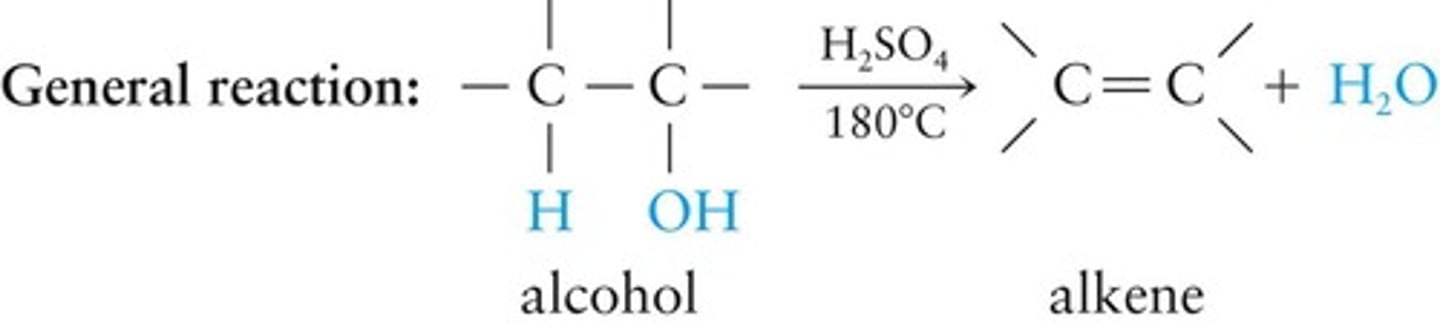
Dehydration Reaction
Reaction in which water is chemically removed from a compound.
Dehydration Reaction temperature
At 140°C, the main product is an ether.
Elimination reaction
Two or more covalent bonds are broken and a new multiple bond is formed.
Alcohol oxidation agents
Reactions with oxidizing agents such as K2Cr2O7 and KMnO4.
Primary alcohol
Oxidizes to aldehyde and then to carboxylic acid.
Secondary alcohol
Oxidizes to ketone.
Phenol
C6H5OH; a weak acid with a melting point of 41°C.
Phenols
Compounds that can react with bases to form salt and are used as disinfectants and antioxidants.
Naming Ethers - IUPAC
The method of naming ethers by naming the smaller R group as an alkoxy group and replacing the -yl ending with -oxy.
Cyclic Ethers
Ethers that are part of a heterocyclic ring, which includes atoms other than carbon.
Properties of Ethers
Ethers are much less polar than alcohols, more soluble in water than hydrocarbons, have low boiling and melting points, are inert, and highly flammable.
Thiol
A compound containing an —SH group, characterized by a strong and offensive odor.
Thiol Reactions - Oxidation
The process that forms disulfide (—S—S—) linkages, important structural features of some proteins.
Thiol Reactions - Reducing Agent
Oxidation reactions of thiols can be reversed with a reducing agent such as H2.
Thiol Reactions - Heavy Metal Ions
Thiols react with heavy metal ions (Pb2+, Hg2+) to form insoluble compounds that can have adverse biological effects.
Polyfunctional Compound
A compound with two or more functional groups that determine its chemical properties.