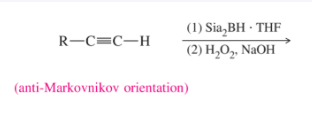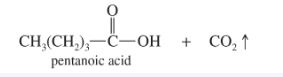Alkynes
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
Alkyne Deprotonation
NaNH₂; forms acetylide ion.
Acetylide SN2 Reactions
Acetylide ion + 1° alkyl halide (Cl, Br, I);
Acetylide with 2° Alkyl Halides
Acetylide ion + 2° alkyl halide; E2 elimination favored; forms alkene.
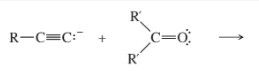
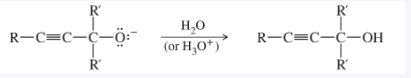
Acetylide Addition to Carbonyls
Acetylide ion + aldehyde or ketone; then H₃O⁺ workup; forms propargylic alcohol.
BuLi Deprotonation + Epoxide Attack
BuLi then epoxide; then H₃O⁺ workup; solvent: THF; SN2 opening at less substituted carbon.
Alkyne Catalytic Hydrogenation
H₂ with Pd, Pt, or Ni; solvent: EtOH; full reduction to alkane.
Lindlar’s Catalyst (Partial Hydrogenation)
H₂ with Pd/BaSO₄ and quinoline; solvent: EtOH; cis alkene only.
Metal–Ammonia Reduction (Na/NH₃)
Na in NH₃(l); trans alkene formation.
Alkyne Addition to Halogens (X₂)
Cl₂ or Br₂; solvent: CCl₄; 1 eq → dihalide, 2 eq → tetrahalide.
Hydrogen Halide Addition to Alkynes (HX)
HCl, HBr, or HI; Markovnikov addition; forms geminal dihalide.
Radical HBr Addition to Alkynes
HBr + ROOR; anti-Markovnikov addition; stops at alkene stage.
Mercuric Ion–Catalyzed Hydration of Alkynes
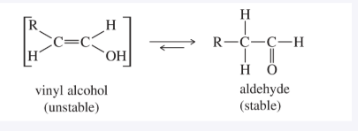
HgSO₄, H₂SO₄, H₂O; Markovnikov hydration gives enol which tautomerizes to ketone