L2 - Ketones and FA Synthesis
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
ketone bodies
low blood sugar / diabetes / fasting
liver synthesizes, extrahepatic tissues uses for glycolysis
FA break down > ACoA
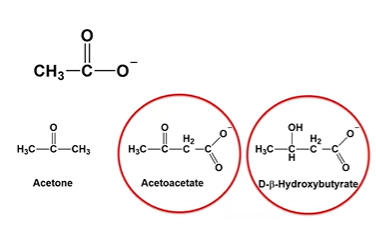
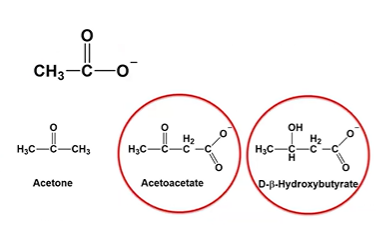
acetoacetate
A primary ketone body that can be converted into acetone or reduced to β-hydroxybutyrate. It’s used by tissues for ATP production via conversion back to acetyl-CoA.
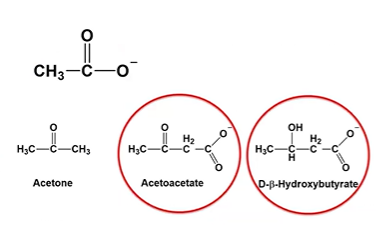
D-B-Hydroxybutyrate
ketogenesis in the liver
*thioLACE ties 2 ACES together, synthetase adds ACoA, lyase lets go ACoA, decarboxylase loses CO2, dehydrogenase reduces it
*TSLAB
thiolase + 2ACoA > + AACoA + CoASH
HMGCoASase + AACoA + ACoA + H2O > HMGCoA
HMGCoALase + HMGCoA > AAc (exported+ ACoA
AAcDClase + AAc > Acetone (exhaled) + CO2
DBHBDHase + AAc + NADH > DBHB (exported)
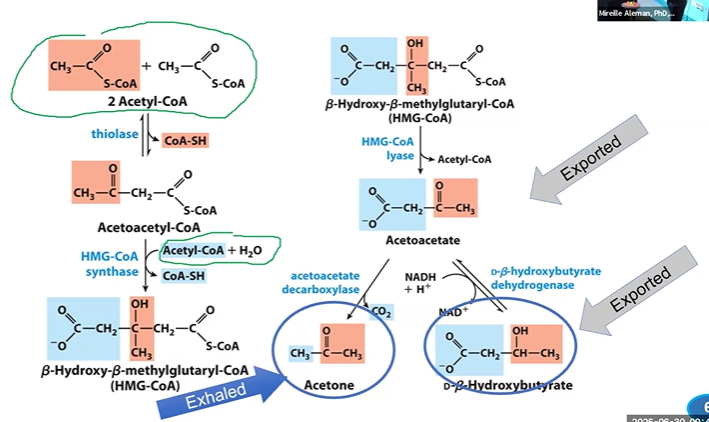
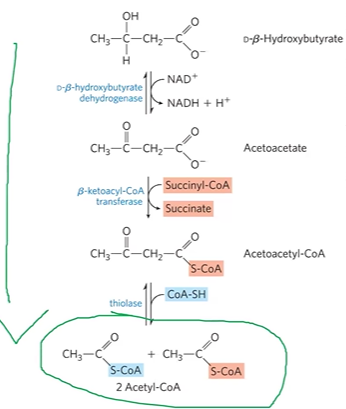
reverse ketogenesis in extrahepatic (non liver) tissues
DBHBDHase + DBHB + NAD+ > AAc
forms acetoacetate via DHase (reverse of previous step)
BKACoATase + SCoA + AAc >AACoA + Succ
forms acetoacetylCoA and succinate using succCoA and BKACoATrase
Thiolase + AACoA + CoASH > 2ACoA
forms 2ACoA using CoASH and thiolase
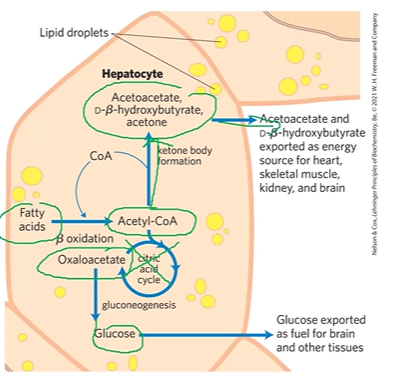
fasting and starvation
FA in lipid droplets in hepatocytes go thru Boxidation > ACoA
ACoA should > Krebs but cant
ACoA > KB formation which supplies CoA for Boxidation (pos feedback)
AAc, DBHB, acetone > energy for body / exhaled
FA Synthesis
make long chain FA, in liver and fat
highly regulated and expensive
FA Synthesis overall reaction
8ACoA + 14NADPH + 7ATP + 14H+ >
Palmitate C16 + 14NADP+ + 8CoA + 7ADP + 7Pi + 6H2O
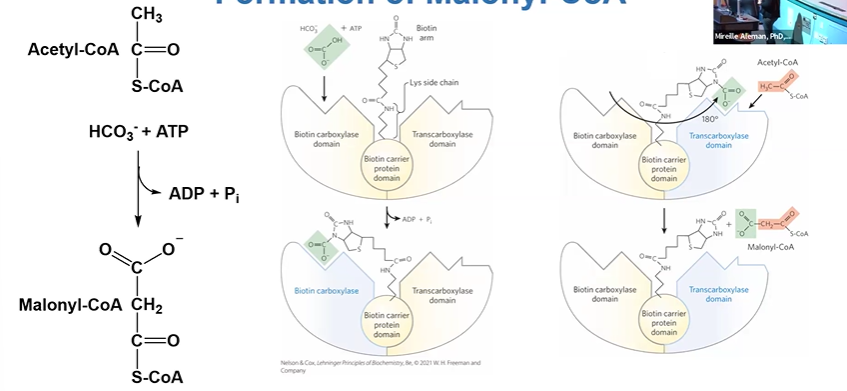
formation of malonylCoA
cat by ACC (Acetyl CoA Carboxylase), needs biotin as CO2 carrier
ACC + ACoA + HCO3- + ATP > MCoA +ADP + Pi
central to regulation of FA synthesis
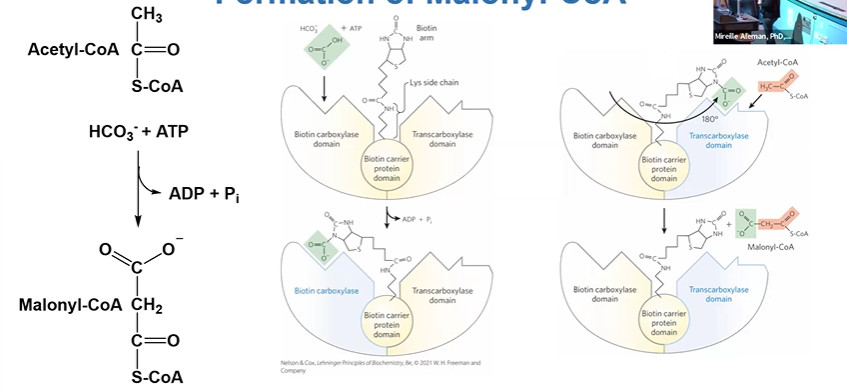
ACC
has biotin carboxylase, transcarboxylase, and biotin carrier protein domains
regulated by covalent modifications and allosteric control
HCO3 binds BClase domain
biotin arm swings over to BClase
180 turn to TClase domain where ACoA is bound
CO2 from HCO3 + ACoA > MCoA leaving enzyme
FA Synthase enzyme
KS = synthase, thiol group
MAT = transferase
DH = DHatase
ER = reductase
KR = reductase, has ACP
ACP = acyl carrier protein, thiol group, activate Carbonyl groups
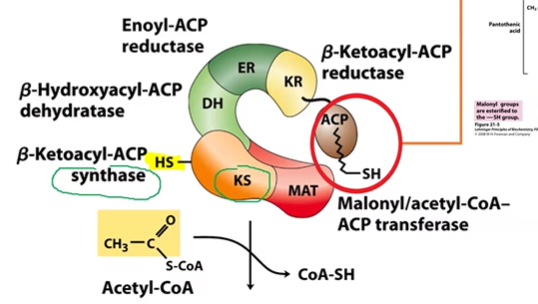
activation of FA synthesis part 0
KS primed with 1ACoA on thiol group, product CoASH
MCoA loaded on ACP, product CoASH, both thiol groups now occupied and nearby
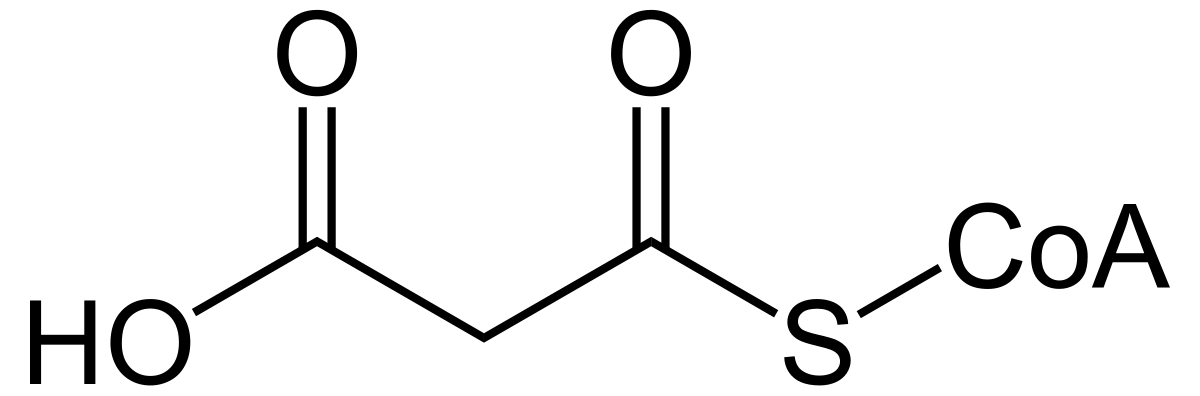
Malonyl CoA
CO2-CH2-C=O-SCoA
A 3-carbon intermediate formed by ACC; provides 2-carbon units for elongation in fatty acid synthesis. Also inhibits carnitine shuttle to prevent simultaneous β-oxidation.
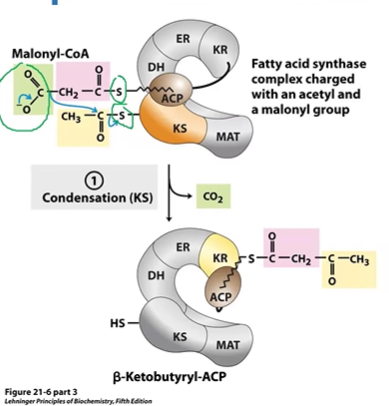
FA S Step 1 Condensation
MCoA CO2 attacks Carbonyl on KS > BKB-ACP (4C) + CO2
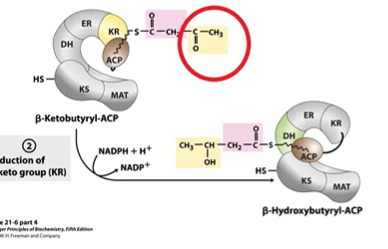
FA S Step 2 Reduction
ACP carries to DH are + NADPH, end =O > OH
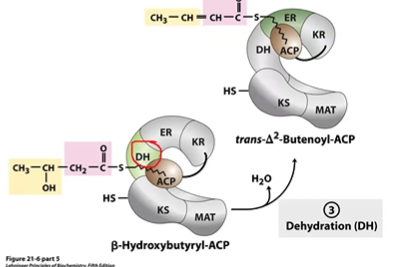
FA S Step 3 Dehydration
BHB-ACP to DH> tdelta2BE-ACP
-OH > C=C
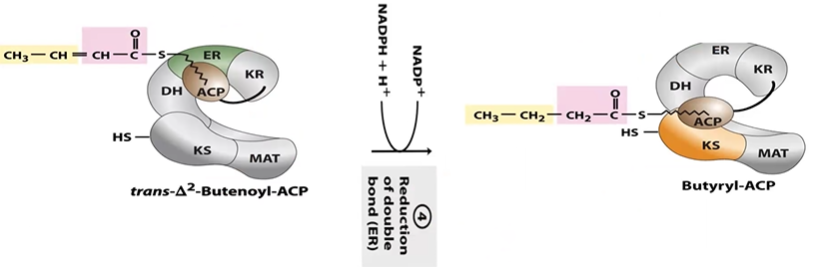
FA S Step 4 Reduction
tD2BE-ACP + NADPH > By-ACP (4C sat. chain)
done in ER
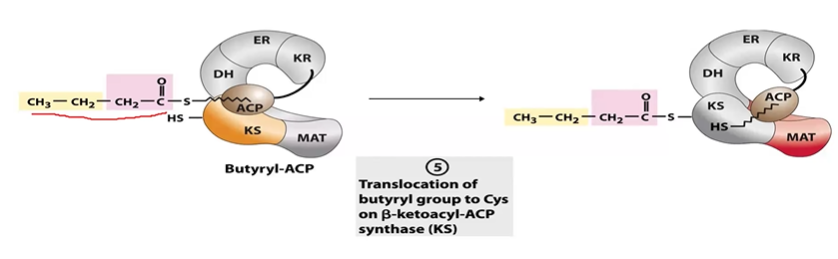
FA S Step 5 Transfer
ACP 4C > KS 4C so ACP can restart
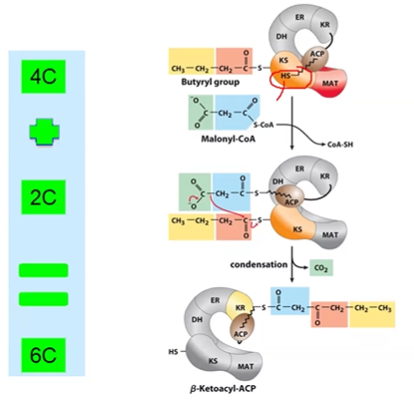
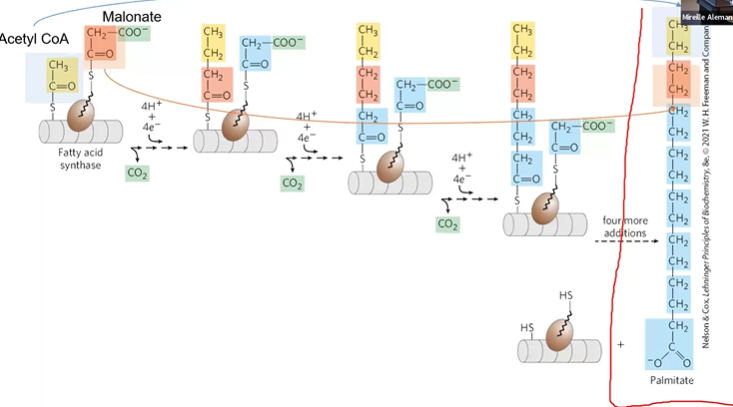
FA S Extension
repeat process until 16C carbon
thioesterase Hlyzes final bond to restart
FA Synthesis Overal Process
*MASRD = malonyl, activation by ACoA, condensation by KS, reduction by KR, dehydration by DH,
(malonyl formation and activation) 1. Condensation 2. Reduction 3. Dehydration 4. Reduction 5. Translocation 6. Extension
1) 7ACoA + 7CO2 + 7ATP > 7MCoA + 7ADP + 7Pi
2) ACoA + 7MCoA + 14NADPH + 14H+ > Palmitate + 7CO2 + 8CoA + 14NADP+ + 6H2O
7ATP + 14NADPH + 14H+ > Palmitate + 7ADP + 7Pi + 14NADP+ + 6H2O
thioesterase
Hlyze thioester group
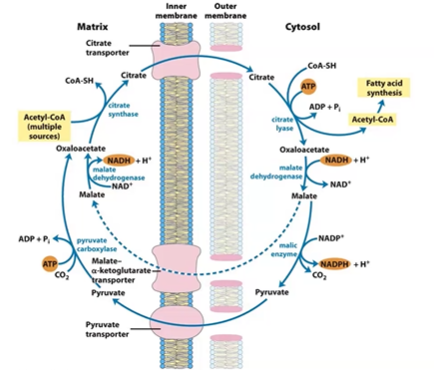
acetate transport
FA is in cytoS, ACoA is stored in mitoC cause Box / PDH
no ACoA transporter, only citrate
pull cit out of CAC in mitoC cause body is high energy
Long Chain FA
>16C, must be from diet
elongated by elongases
or desaturation by desaturases
primary metabolic source of reducing power required for FA synthesis and desaturation
oxidative phase of PPP, bc NADPH is red agent in FA synthesis
regulation of FA synthesis
metabolic intermediates regulate ACoAClase
Ggon, epi, AMP, PalmitoylCoA all inhibit
citrate activates MCoA inhibits carnitine acyltransferase
CAT1 carnitine acyltransferase I inhibited by
MalonylCoA
regulation of FA Boxidation and synthesis
Synthesis
ACC Plated by PKA to inactive
ACC DP by Ptase to active, which makes ACoA > MCoA
glucgaon DA PKA, insulin A Ptase
Oxidation
MCOA inhib CAT1 FACoA > FAcarnitine
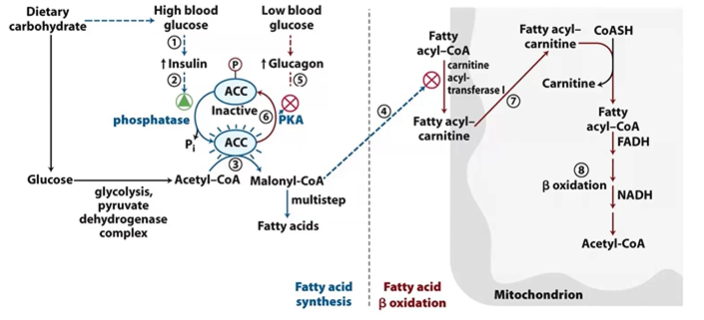
control by glucagon
binding of glucagon to plasma receptor in subsequent P of enzymes by kinases
ACoAClase inhibited, dec FA Synthesis
HSL activated, inc stored fat mobilization
PDHase inhibited, inc gluconeogenesis
control by insulin
binding to plasma receptor in DPlation of enzymes by PPPatases
ACoAClase activated, inc FA Synthesis
HSL inhibited, decstored fat mobilization, inc stored fats
PDHase activated, dec gluconeogenesis, inc glycolysis
ACoA carboxylase
cat. rate limiting step of FA synthesis, formation of MCoA
allosterically activated by citrate
acetone
A volatile, non-metabolizable ketone body. It is a byproduct of ketogenesis and is exhaled or excreted in urine.
BHB B hydroxybutyrate
A stable, energy-rich ketone body, interconvertible with acetoacetate. Used as a fuel in peripheral tissues, including the brain during prolonged fasting.
thiolase
Catalyzes the condensation of two acetyl-CoA molecules to form acetoacetyl-CoA, an early step in ketogenesis and also important in β-oxidation.
HMGCoA Rase
Key regulatory enzyme in cholesterol synthesis, not ketogenesis. Converts HMG-CoA to mevalonate. Inhibited by statins.
HMGCoA Lyase
Catalyzes the cleavage of HMG-CoA to acetoacetate and acetyl-CoA during ketogenesis in liver mitochondria.
ACC acetylCoA Carboxylase
Rate-limiting enzyme of fatty acid synthesis. Converts acetyl-CoA to malonyl-CoA using ATP and biotin. Inhibited by palmitoyl-CoA, activated by citrate and insulin.
biotin
A coenzyme (vitamin B7) required by carboxylases like ACC to transfer CO₂ during carboxylation reactions.
FAS fatty acid synthetase
A multi-enzyme complex that performs the repeated addition of malonyl-CoA to a growing fatty acid chain.
ACP
Part of FAS; it holds the growing fatty acid chain and intermediates during elongation.
acyl carrier protein
OA oxaloacetate
Combines with mitochondrial acetyl-CoA to form citrate (via citrate synthase) for transport.
citrate transporter
Transports citrate from mitochondria into cytosol, where citrate is cleaved to release acetyl-CoA and OAA.
malate aKG transporter
Moves malate or α-ketoglutarate across mitochondrial membranes to help regenerate NADPH or shuttle carbon skeletons.
pyruvate transporter
Brings pyruvate from cytosol into mitochondria, completing the cycle for citrate-pyruvate shuttle (OAA + NADH → malate → pyruvate).