MEDCHEM
0.0(0)
Card Sorting
1/100
There's no tags or description
Looks like no tags are added yet.
Last updated 2:33 AM on 5/16/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
101 Terms
1
New cards
Which of the following statements is true in relation to phase II metabolism?
Phase II metabolism involves the oxidation of drugs by cytochrome P450 enzymes.
Phase II metabolism usually leads to a drug molecule with a higher molecule weight.
Phase II metabolism typically results in the formation of more hydrophobic drug molecules.
Phase II metabolism involves the hydrolysis of esters using esterases.
Phase II metabolism usually leads to a more active drug molecule.
Phase II metabolism involves the oxidation of drugs by cytochrome P450 enzymes.
Phase II metabolism usually leads to a drug molecule with a higher molecule weight.
Phase II metabolism typically results in the formation of more hydrophobic drug molecules.
Phase II metabolism involves the hydrolysis of esters using esterases.
Phase II metabolism usually leads to a more active drug molecule.
Phase II metabolism usually leads to a drug molecule with a higher molecule weight.
2
New cards
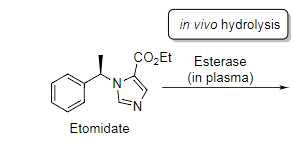
Phase I Metabolism Metabolism by hydrolysis: esterases and peptidases can potentially
hydrolyse any ester or amide
3
New cards
Phase I Metabolism Metabolism by hydrolysis:
Etomidate is a
Etomidate is a
short-acting intravenous anesthetic,
4
New cards
Phase I Metabolism Metabolism by hydrolysis:
Etomidate is inactivated in the
Etomidate is inactivated in the
blood plasma
5
New cards
Phase I Metabolism Metabolism by hydrolysis:
Etomidate is inactivated in the blood plasma by the actions of
Etomidate is inactivated in the blood plasma by the actions of
esterases.
6
New cards
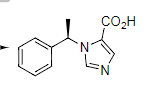
Phase I Metabolism Metabolism by hydrolysis:
The enzyme hydrolyses the ester to the corresponding
The enzyme hydrolyses the ester to the corresponding
carboxylic acid
7
New cards
Phase I metabolism is carried out by various enzymes -
cytochrome P450s (CYPs), monoamine oxidase, \n flavin monooxygenase, xanthine oxidase, esterases/peptidases (hydrolysis)
8
New cards
Phase II Metabolism conjugation: \n Glucuronidation/sulphation occur typically at ? groups
phenol, alcohol and carboxylic acid
9
New cards
Phase I metabolism, products are often more
reactive
10
New cards
Phase II Metabolism conjugation:
The products of conjugation are typically higher in ? and less ? than the parent compounds
The products of conjugation are typically higher in ? and less ? than the parent compounds
MW, reactive
11
New cards
Phase II Metabolism conjugation:
The process is ? mediated (transferases)
The process is ? mediated (transferases)
enzyme
12
New cards
Phase II Metabolism conjugation:
The ? group may have been already present in the drug, or added during phase I metabolism
The ? group may have been already present in the drug, or added during phase I metabolism
OH
13
New cards
Phase I Metabolism Oxidative metabolism Reactions mediated by
CYPs
14
New cards
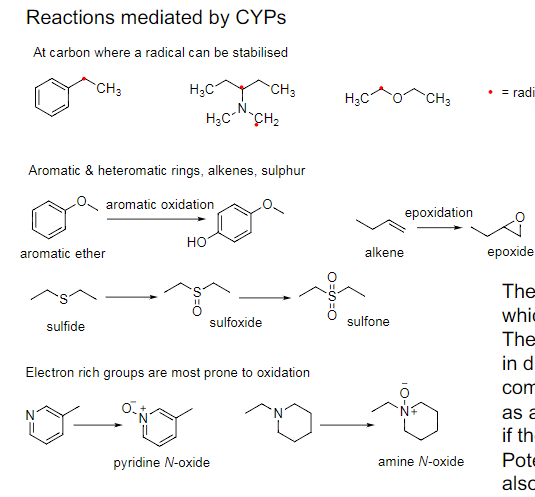
Phase I Metabolism Oxidative metabolism PLACES:
At carbon where a radical can be stabilised
Aromatic & heteromatic rings, alkenes, sulphur
Electron rich groups are most prone to oxidation
Aromatic & heteromatic rings, alkenes, sulphur
Electron rich groups are most prone to oxidation
15
New cards
Phase I EVENTS –
oxidation, reduction, hydrolysis
16
New cards
Phase II EVENTS –
conjugation
17
New cards
In relation to structure activity relationships for a particular drug molecule, changing a secondary amide to a secondary amine is most likely to reveal that:
the carbonyl is not essential for activity
ionic interactions are essential for activity
the nitrogen is essential for activity
electrostatic interactions are not important for binding
the nitrogen is not essential for activity
van der Waals interactions are not playing a significant part in drug binding
the carbonyl is not essential for activity
ionic interactions are essential for activity
the nitrogen is essential for activity
electrostatic interactions are not important for binding
the nitrogen is not essential for activity
van der Waals interactions are not playing a significant part in drug binding
the carbonyl is not essential for activity
18
New cards
DRUGS BIND TO TARGET van der Waals Interactions
KNOWN AS
KNOWN AS
London dispersion forces
19
New cards
DRUGS BIND TO TARGET van der Waals Interactions \n These are ? interactions
short range, relatively weak
20
New cards
DRUGS BIND TO TARGET van der Waals Interactions
can be extremely important for ?
can be extremely important for ?
binding
21
New cards
DRUGS BIND TO TARGET van der Waals Interactions
Require ? between drug and target
Require ? between drug and target
close contact
22
New cards
An important feature of enzymes is their high substrate specificity and this is due to a series of non-covalent enzyme-substrate interactions:
• Electrostatic \n • Hydrogen bonding \n • Non-polar (Van der Waals) interactions \n • Hydrophobic
23
New cards
electrostatic/ionic bonds:
? intermolecular interaction
? intermolecular interaction
strongest
24
New cards
electrostatic/ionic bonds:
take place between
take place between
groups of opposite charge
25
New cards
electrostatic/ionic bonds:
stronger interactions occur in
stronger interactions occur in
hydrophobic environments
26
New cards
electrostatic/ionic bonds:
very important as drug
very important as drug
enters binding site
27
New cards
Amines Prevents nitrogen acting as a ?, due to resonance
HBA
28
New cards
Amines Prevents nitrogen acting as a HBA, due to ?
resonance
29
New cards
Carboxylic acids
May exist as carboxylate ion, where there is the possibility of
May exist as carboxylate ion, where there is the possibility of
ionic interactions or very strong H-bond acceptor
30
New cards
The carbonyl oxygen has greater electron density and is less hindered than the alkoxy oxygen, so will typically be a better
HBA
31
New cards
The relevance of the carbonyl group could be tested by
making the equivalent ether analogue
32
New cards
The carbonyl oxygen has greater ? and is less ? than the alkoxy oxygen, so will typically be a better HBA
electron density, hindered
33
New cards
carbonyl
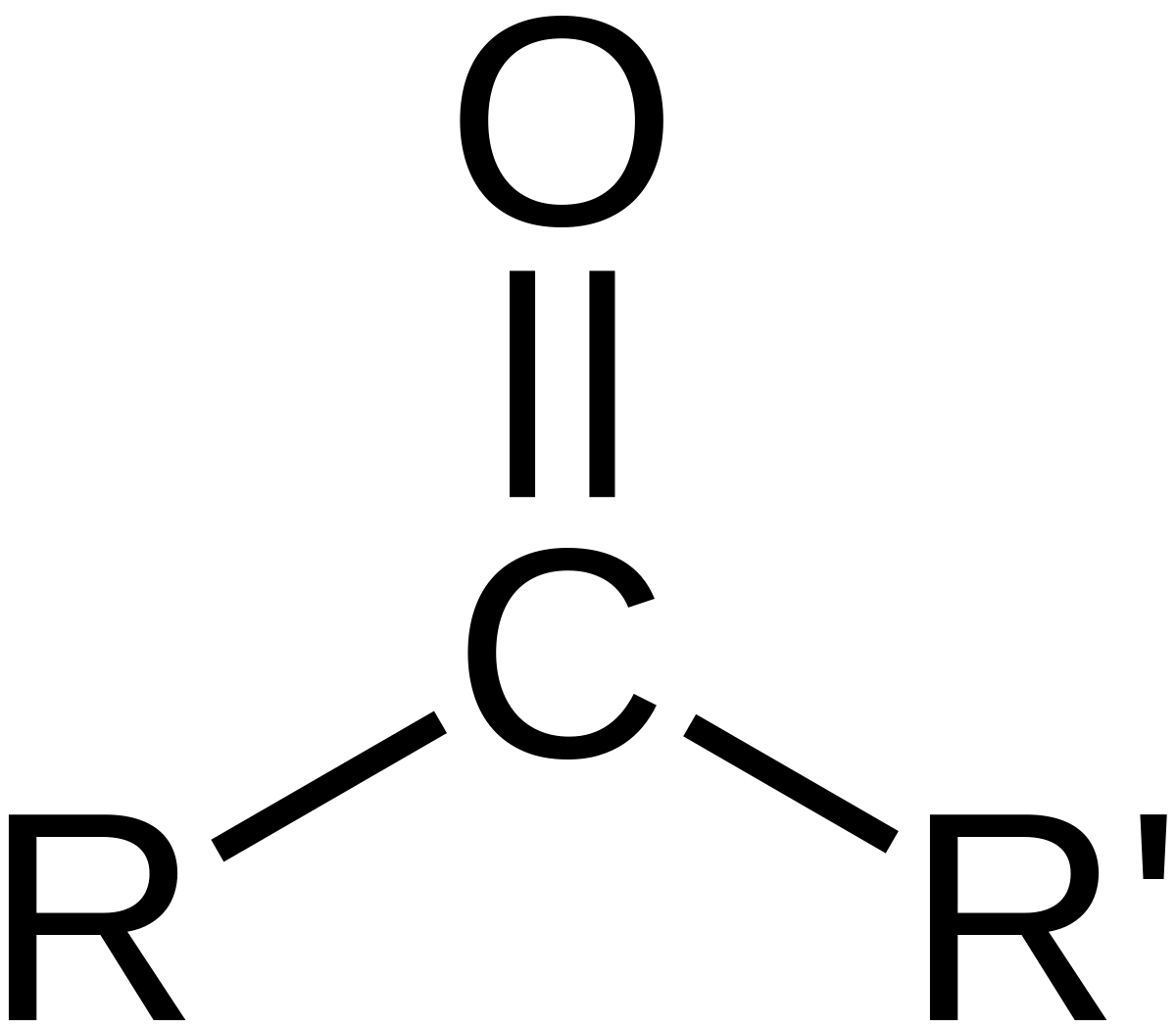
34
New cards
Ketones and aldehydes
Carbonyl to alcohol
35
New cards
aldehyde
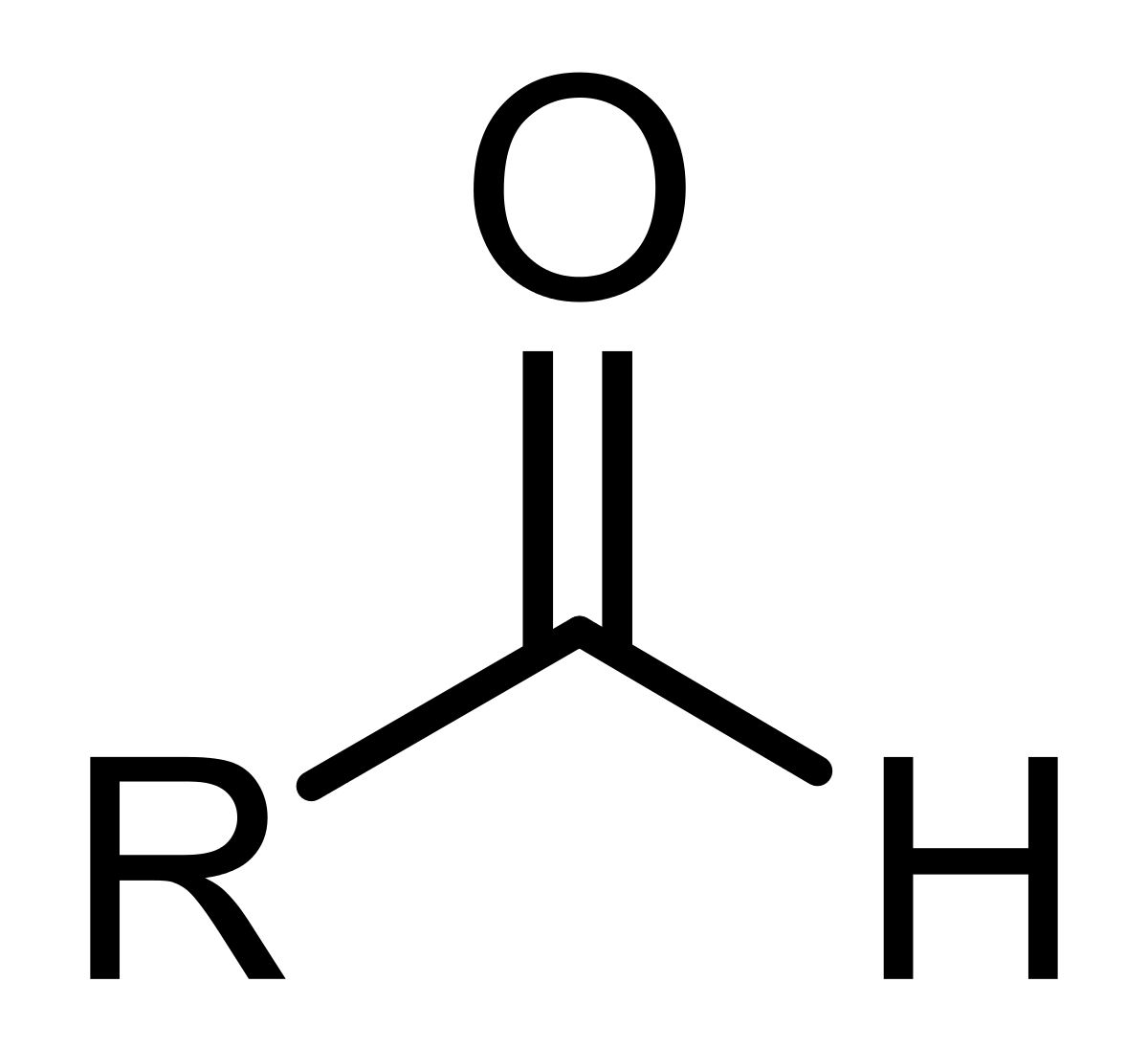
36
New cards
ketone
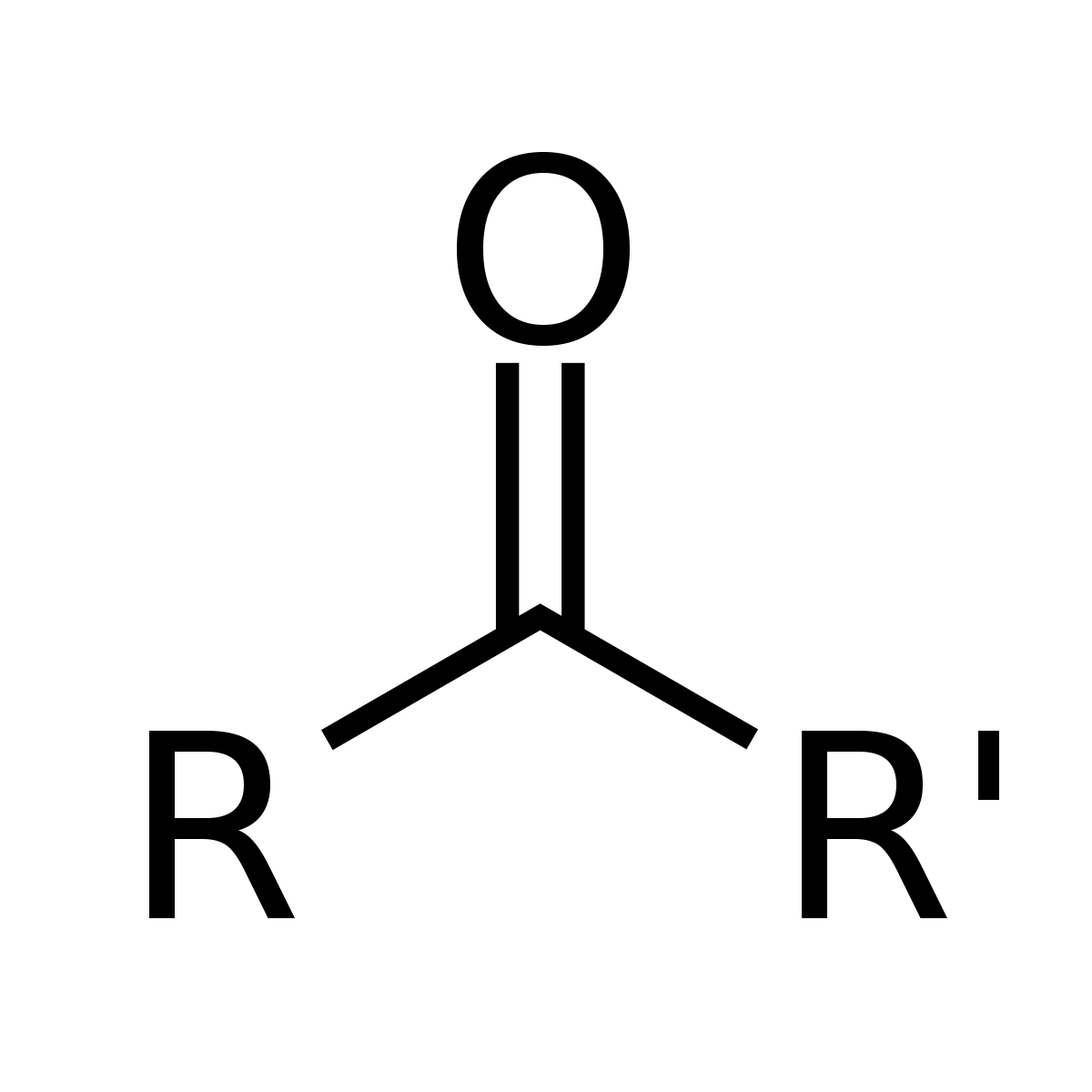
37
New cards
Amides are very common in drugs (e.g. ?) and ? amides are the most common
peptides, secondary
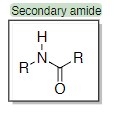
38
New cards
Relatively easy to form primary and secondary amides from
primary/secondary amines
39
New cards
Which of the following statements is false?
Peptide bonds are rigid and planar .
Peptide bonds can be broken through the action of peptidases.
A peptide bond is formed when the carboxyl group of one molecule reacts with the amino group of another molecule.
Peptide bonds link amino acids together in a peptide chain.
Peptide bonds are key features of drug targets.
Peptide bonds are unstable and readily hydrolysed in water.
Peptide bonds are rigid and planar .
Peptide bonds can be broken through the action of peptidases.
A peptide bond is formed when the carboxyl group of one molecule reacts with the amino group of another molecule.
Peptide bonds link amino acids together in a peptide chain.
Peptide bonds are key features of drug targets.
Peptide bonds are unstable and readily hydrolysed in water.
Peptide bonds are unstable and readily hydrolysed in water.
40
New cards
Peptide bonds can be broken through the action of
peptidases
41
New cards
A peptide bond is formed when
the carboxyl group of one molecule reacts with the amino group of another molecule.
42
New cards
Peptide bonds link amino acids together in a
peptide chain.
43
New cards
Peptide bonds are key features of
drug targets
44
New cards
Peptide bonds are
rigid and planar
45
New cards
Proteases are enzymes that
cleave proteins and peptides
46
New cards
Substrate binding: the side-chain of the amino acid residue immediately before the scissile peptide bond can
bind to the recognition site on the enzyme
47
New cards
Protonation: His57 donates a proton to the amide nitrogen of the substrate, allowing
the release of the C- terminal portion of the peptide as a free peptide
48
New cards
G protein These receptors act like an inbox for cellular messages –
proteins, peptides, lipids, sugar, light energy
49
New cards
peptide bonds join the ? together to form ?, which fold into a ?(perhaps an enzyme)
amino acids, peptides, protein
50
New cards
Drug Targets- Enzymes
Primary Structure –
Primary Structure –
Peptide bond
51
New cards
Drug Targets- Enzymes
Secondary structure refers to
Secondary structure refers to
the spatial arrangement of amino acid residues that \n are near one another in the linear sequence
52
New cards
Polypeptide chains can fold into
regularly repeating structures, α
\-helices and β-sheets
\-helices and β-sheets
53
New cards
X-ray crystallography is related to:
electron ionization
all answers are correct
photon reflections
magnetic field
ion abundance
electron ionization
all answers are correct
photon reflections
magnetic field
ion abundance
photon reflections
54
New cards
Phytochemistry \n (Some) methods used: \n
•Thin layer chromatography (TLC) \n •Gel (column) chromatography \n •Gas chromatography (GC) \n •Mass spectrometry \n •Nuclear magnetic resonance \n • X-ray crystallography
55
New cards
Crystallography \n The information that you get are based on the analysis of the
diffraction patterns that emerge from a sample that is targeted by a beam of \n some type (like X-ray).
56
New cards
Diffraction is caused by
electron clouds:
57
New cards
the higher the atomic number of an element, the
larger it’s electron clouds are!
58
New cards
xray crystallography can determine
Stereochemistry \n Bond length \n Distance between atoms
59
New cards
Phytochemistry is the study of
phytochemicals produced in plants, describing \n the isolation, purification, identification, and structure elucidation of the large \n number of secondary metabolic compounds found in plants.
60
New cards
Magnetic field is related to
NMR.
61
New cards
Ion abundance and electron ionization are related to
mass spectrometry.
62
New cards
The toxicity of *Atropa belladonna* is due to:
none of the answers is correct
the function of atropine as an antagonist of gamma-butyric acid
the function of atropine as an agonist of acetylcholine
the function of atropine as an antagonist of acetylcholine
the function of atropine as an agonist of gamma-butyric acid
none of the answers is correct
the function of atropine as an antagonist of gamma-butyric acid
the function of atropine as an agonist of acetylcholine
the function of atropine as an antagonist of acetylcholine
the function of atropine as an agonist of gamma-butyric acid
the function of atropine as an antagonist of acetylcholine
63
New cards
atropine extracted from belladonna is used in \n ? as a ?, to temporarily ? the ciliary muscle of \n the eye; and as a ?, to ? the pupils
ophthalmology, cycloplegic, paralyze, mydriatic, dilate
64
New cards
atropine acts as an ? of acetylcholine
antagonist
65
New cards
atropine acts as an antagonist of
acetylcholine
66
New cards
? acts as an antagonist of acetylcholine
atropine
67
New cards
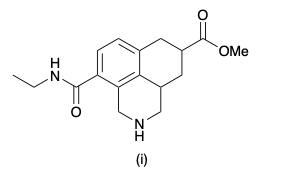
What functional groups does molecule (i) contain?
A primary amine; an ester; a primary amide
A tertiary amine; an ester; a secondary amide
A secondary amine; a carboxylic acid; a secondary amide
A secondary amine; an ester; a secondary amide
A tertiary amine; a carboxylic acid; a primary amide
A primary amine; an ester; a carboxylic acid
A primary amine; an ester; a primary amide
A tertiary amine; an ester; a secondary amide
A secondary amine; a carboxylic acid; a secondary amide
A secondary amine; an ester; a secondary amide
A tertiary amine; a carboxylic acid; a primary amide
A primary amine; an ester; a carboxylic acid
A secondary amine; an ester; a secondary amide
68
New cards
carboxylic acid
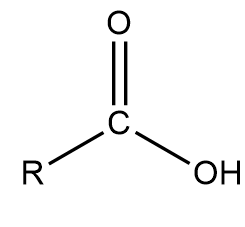
69
New cards
primary secondary tertiary amide
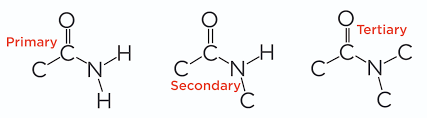
70
New cards
ester
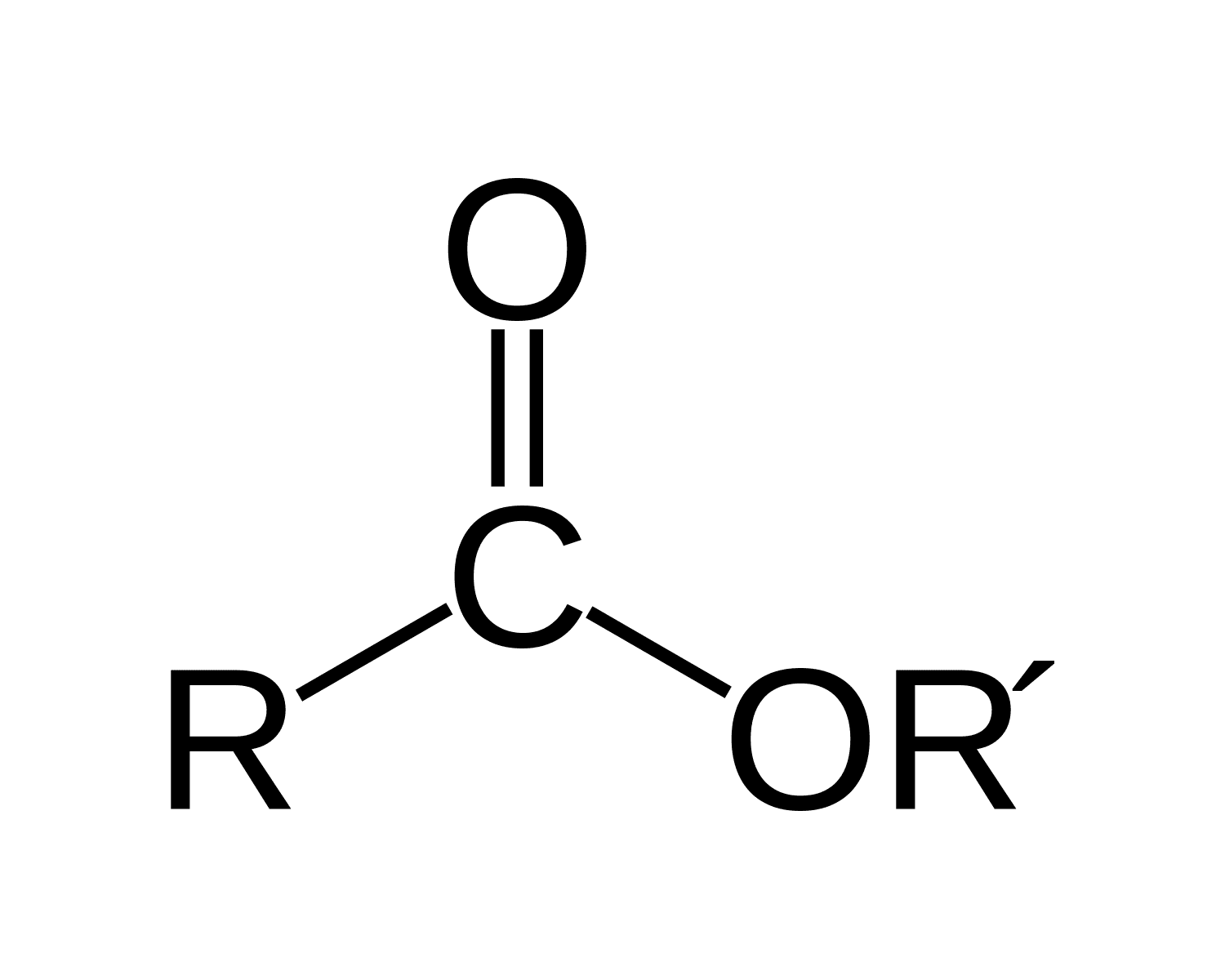
71
New cards
primary, secondary, tertiary amine
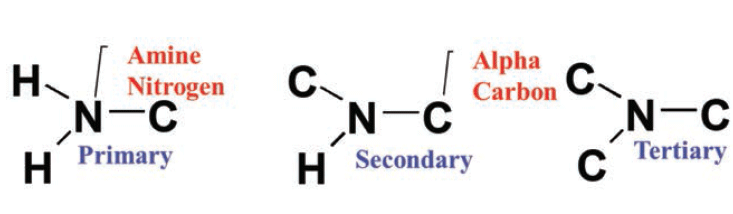
72
New cards
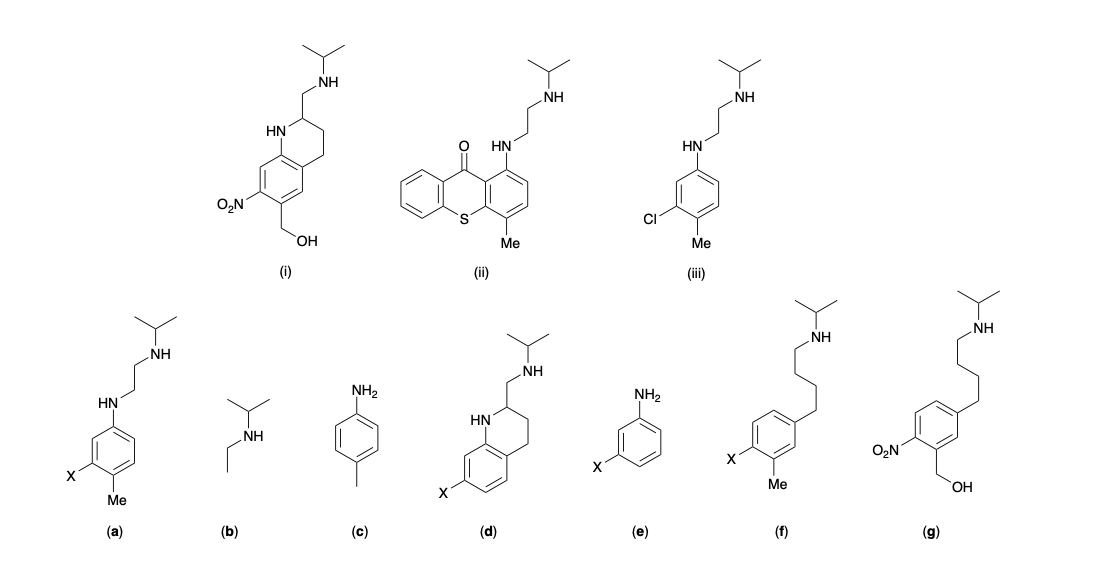
Molecules (i)-(iii) are active anti-parasitic drugs. Which of the following (a)-(g) represents the most likely active pharmacophore for (i)-(iii)?
(a)
(b)
(c)
(d)
(e)
(f)
(g)
(a)
(b)
(c)
(d)
(e)
(f)
(g)
a
73
New cards
Enzymes can reduce the “E factor” because:
all answers are correct
they operate at room temperature
they can overcome the need for organic solvents and other reagents
they can operate at ambient pressure
they are effective at mild pH values
all answers are correct
they operate at room temperature
they can overcome the need for organic solvents and other reagents
they can operate at ambient pressure
they are effective at mild pH values
they can overcome the need for organic solvents and other reagents
74
New cards
The "E factor" in Green Chemistry is defined as
the ratio of kilograms of waste generated per kilogram of product synthesized.
75
New cards
A method used for separating a mixture of substances based on their different boiling points is called:
fractional distillation
thin layer chromatography
gas chromatography
partial solubilisation
fractional extraction
fractional distillation
thin layer chromatography
gas chromatography
partial solubilisation
fractional extraction
fractional distillation
76
New cards
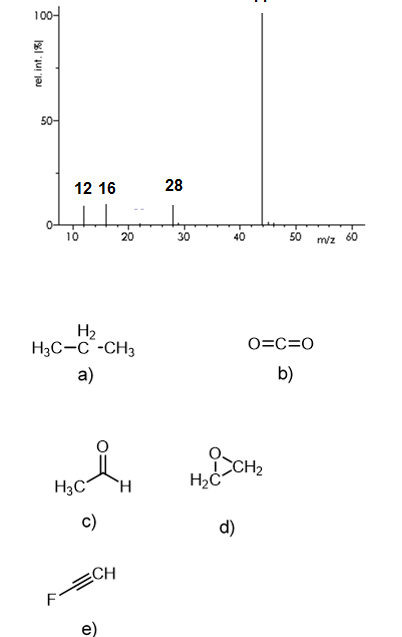
The following mass spectrum is representative of:
a
b
c
d
e
a
b
c
d
e
c ("Fragments": C = 12; O = 16; CO = 28; CO2 = 44)
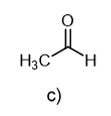
77
New cards
The production of new antibiotics by genetic manipulation of microorganisms is an example of:
red biotechnology
green biotechnology
grey biotechnology
white biotechnology
blue biotechnology
red biotechnology
green biotechnology
grey biotechnology
white biotechnology
blue biotechnology
red biotechnology
78
New cards
A lone pair of electrons is:
always a strong hydrogen bond donor
never a hydrogen bond acceptor
always a weak hydrogen bond donor
never a hydrogen bond donor or acceptor
always a strong hydrogen bond acceptor
sometimes a strong hydrogen bond acceptor
always a strong hydrogen bond donor
never a hydrogen bond acceptor
always a weak hydrogen bond donor
never a hydrogen bond donor or acceptor
always a strong hydrogen bond acceptor
sometimes a strong hydrogen bond acceptor
sometimes a strong hydrogen bond acceptor
79
New cards
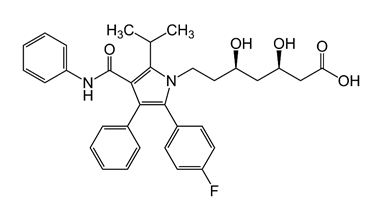
Which chemical functional group is not present in the following structure?
hydroxy group
ester
amide
carboxylic acid
aromatic ring
hydroxy group
ester
amide
carboxylic acid
aromatic ring
ester
80
New cards
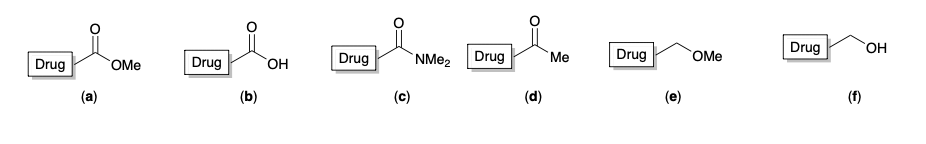
Which one of the following molecules is likely to engage in important ionic binding interactions as a drug enters the target binding site?
(a)
(b)
(c)
(d)
(e)
(f)
(a)
(b)
(c)
(d)
(e)
(f)
b) drug - c = o - OH
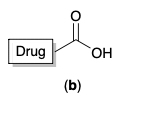
81
New cards
In relation to drug optimisation strategies, which of the following statements is true?
***Ring contraction*** is not an example of a drug optimisation strategy.
***Rigidification*** leads to a drug with a single active conformation.
***Simplification*** involves the systematic removal of functional groups in a drug.
***Simplification*** involves the systematic removal of chiral centres in a drug.
***Ring fusion*** allows the drug molecule to increase its number of active conformations.
***Ring expansion*** is primerally used to increase the molecular weight of a drug molecule.
***Ring contraction*** is not an example of a drug optimisation strategy.
***Rigidification*** leads to a drug with a single active conformation.
***Simplification*** involves the systematic removal of functional groups in a drug.
***Simplification*** involves the systematic removal of chiral centres in a drug.
***Ring fusion*** allows the drug molecule to increase its number of active conformations.
***Ring expansion*** is primerally used to increase the molecular weight of a drug molecule.
***Simplification*** involves the systematic removal of functional groups in a drug.
82
New cards
ring expansion
a chemical reaction that involves the breaking and forming of covalent bonds to increase the size of a cyclic compound.
83
New cards
ring expansion can be achieved through various methods such as
heat, acid catalysis, or the use of specific reagents.
84
New cards
ring fusion
the process of joining two or more rings together to create a new compound with potentially unique properties.
85
New cards
ring fusion This technique is often used to modify the structure of existing drugs in order to
improve their efficacy or reduce their side effects.
86
New cards
rigidification
the process of making something rigid or stiff, often through the use of a hardening agent or by applying pressure.
87
New cards
ring contraction
a cyclic compound undergoes a rearrangement to form a smaller cyclic compound.
88
New cards
One common example of ring contraction is
the conversion of cyclohexanone to cyclopentanone through the use of acid catalysis.
89
New cards
***Simplification*** involves
the systematic removal of functional groups in a drug.
90
New cards
The interaction between tyrosine and phenylanine in a protein is an example of:
disulfide bond
a cation-pi interaction
pi-stacking
a ionic bond
a salt bridge
disulfide bond
a cation-pi interaction
pi-stacking
a ionic bond
a salt bridge
pi-stacking
91
New cards
pi-stacking
non-covalent interaction between aromatic rings, where the pi-electron clouds of the rings overlap and form a stabilizing interaction.
92
New cards
pi stacking is commonly observed in organic molecules, and plays a significant role in various biological processes such as
DNA replication and protein folding.
93
New cards
a cation-pi interaction
non-covalent interaction between a positively charged ion (cation) and an aromatic molecule with a pi electron cloud.
94
New cards
a cation-pi interaction This interaction is important in various biological processes, such as
protein-ligand binding and DNA-protein interactions.
95
New cards
disulfide bond
covalent bond between two sulfur atoms in two different amino acids within a protein molecule.
96
New cards
disulfide bond helps
stabilize the protein's three-dimensional structure.
97
New cards
By definition, an oral drug is always 100% bioavailable.
True
False
True
False
false
98
New cards
In an aqueous environment, an ionic interaction is most likely to take place between:
a protonated amine and a carboxylate
a protonated amine and an alcohol
a protonated amine and an ester
a carboxylate and an alcohol
a carboxylate and an ester
an amide and a carboxylate
a protonated amine and a carboxylate
a protonated amine and an alcohol
a protonated amine and an ester
a carboxylate and an alcohol
a carboxylate and an ester
an amide and a carboxylate
a protonated amine and a carboxylate
99
New cards
One of the natural amino acids is not chiral because:
it is not superimposable with its mirror image
it does not have a mirror image
it is too small
it is not polar
two identical substituents are attached to the central carbon
it is not superimposable with its mirror image
it does not have a mirror image
it is too small
it is not polar
two identical substituents are attached to the central carbon
two identical substituents are attached to the central carbon
100
New cards
When ?, the substance is not chiral.
two identical groups are attached to a tetrahedral carbon