AP Biology Cell Membranes and Transport
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
Cell Theory
All organisms are composed of cells
Cell are the basic units of life
Cells arise only from pre-existing cells
Prokaryote vs Eukaryote Cell Size
Prokaryote: 1-10μm diameter
Eukaryote: 10-100μm diameter
Cell Size Limitation
Most cells are relatively small due to reliance on diffusion of substances in and out of cells
Diffusion Rates
Affected by:
SA/V ratio
Temperature
Concentration Gradient
Distance
Surface Area/Volume Ratio
Good for it to be big
As cell size increases, volume grows faster than surface area, making exchange of materials less efficient. High SA:V allows faster diffusion of nutrients, gases, and wastes
Small Cell Size
Most cells are small
Skeletal muscle cells are large but have multiple nuclei
Some cells are long and skinny to transmit signals faster like nerve cells
Cell Membrane Functions
Holds in contents
Regulates what goes in and out of the cell
Cell Membrane Structure
Made of phospholipids
Phosphate head (hydrophilic)
Fatty acid tail (hydrophobic)
Forms a bilayer where only certain molecules can get through
Plasma Membrane
The boundary that separates a cell from its surroundings
Exhibits selective permeability and allows some substances to cross it more easily than others
Amphipathic Molecules
Contain both hydrophobic and hydrophilic parts
Ex. Phospholipids
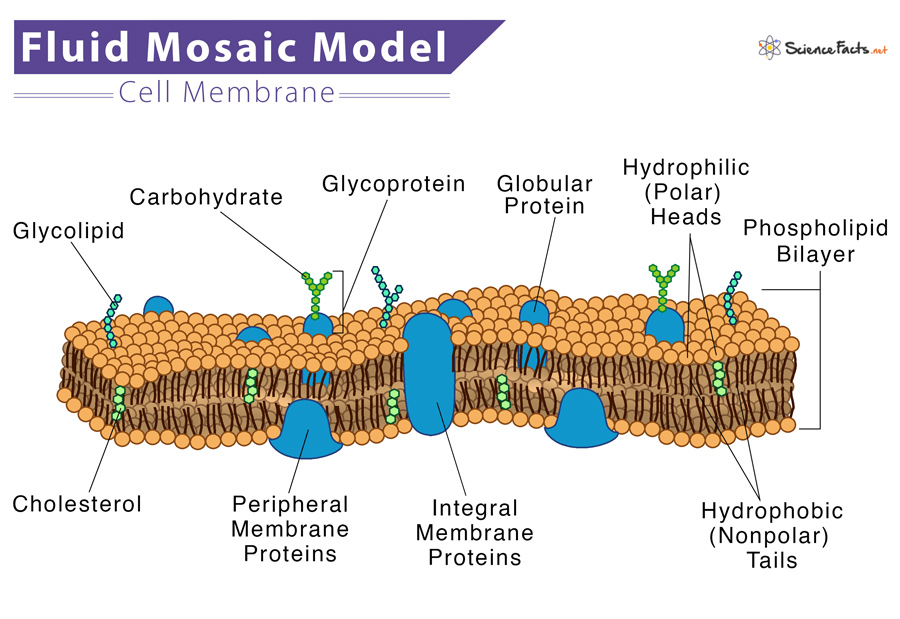
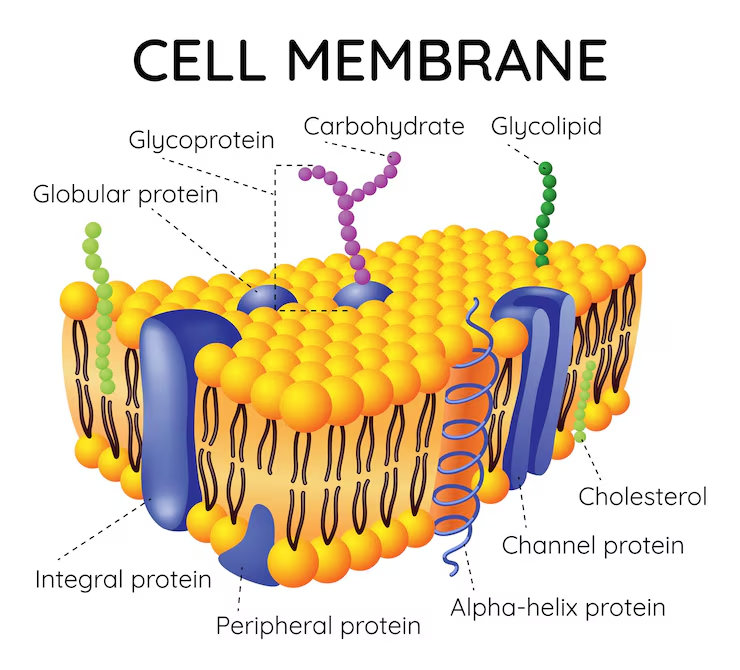
Fluid Mosaic Model
States that a membrane is a fluid structure (phospholipids and unanchored proteins can move around) with a mosaic of various proteins embedded in it
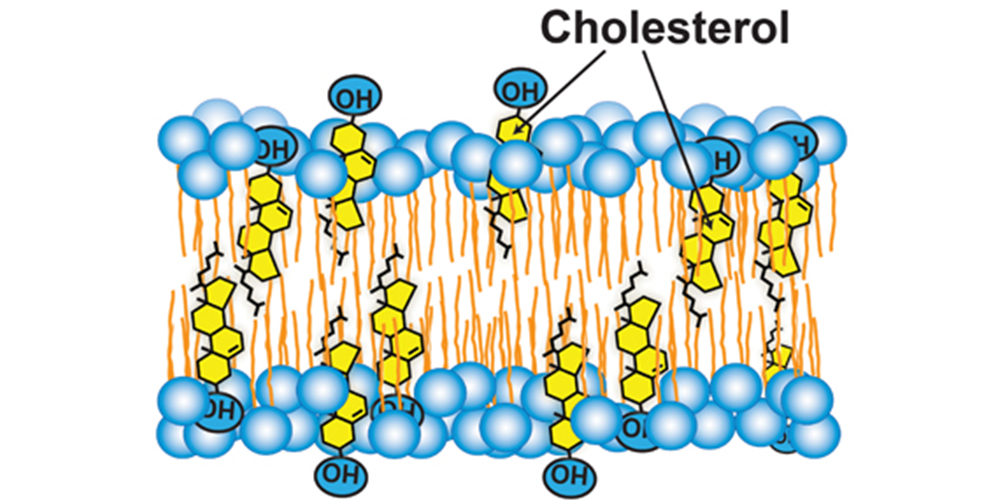
Cholesterol
A steroid
Keeps the phospholipids from locking together/affects membrane fluidity
At warm temps (37°C-body temp), cholesterol restricts movement of phospholipids
At cool temps, it protects fluidity by preventing tight packing
Proteins
Help move things across the membrane
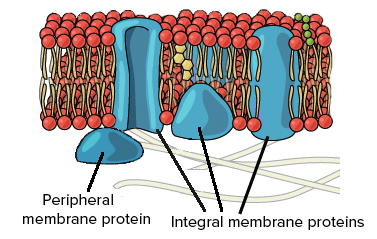
Transmembrane/Integral Protein
Moves things across
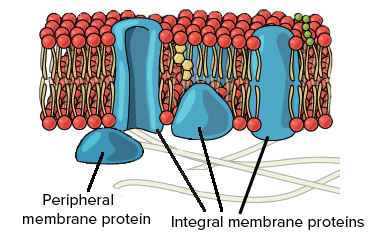
Peripheral Protein
Attached to the outside of inside
Carbohydrates
Helps cells identify each other
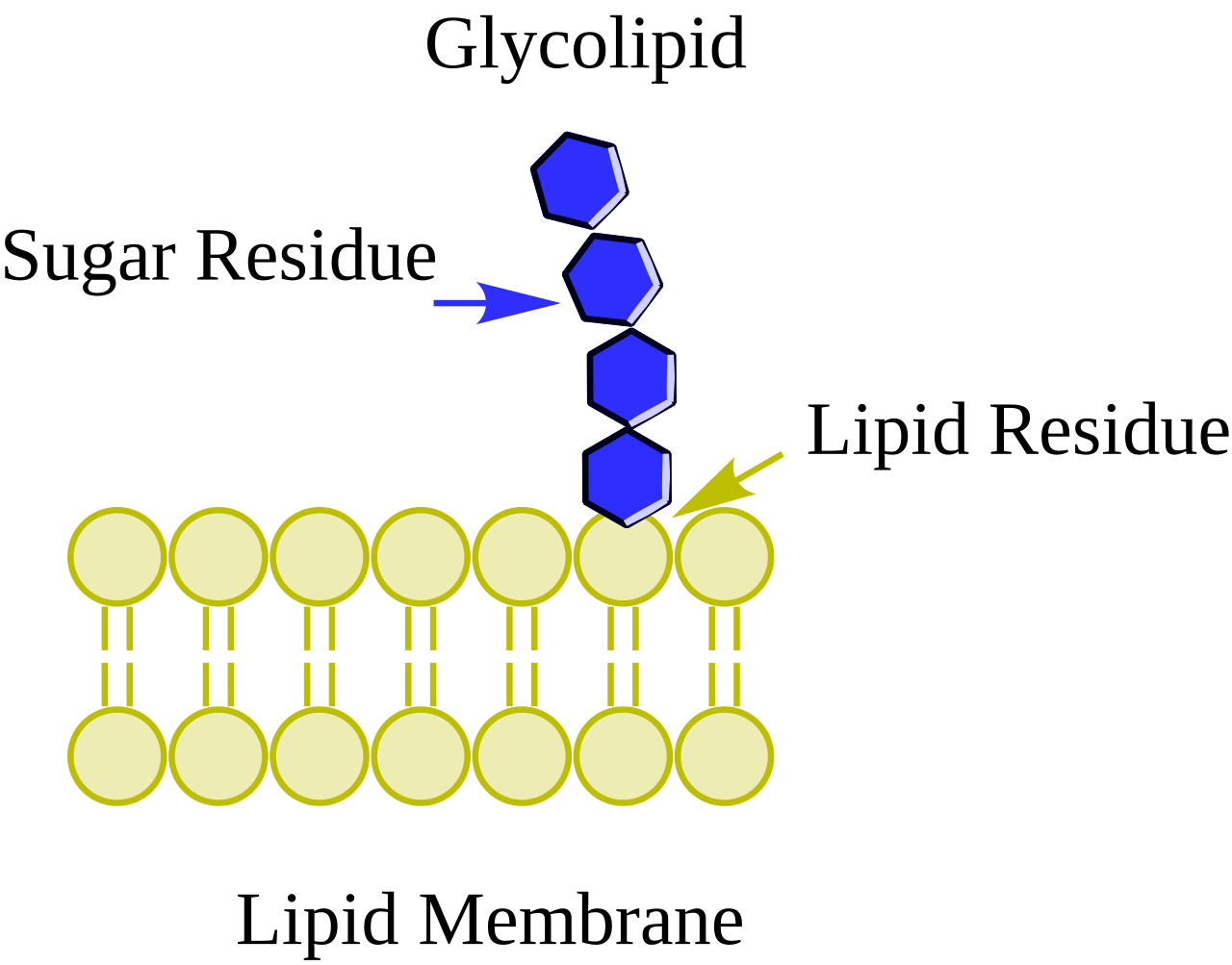
Glycolipid
Carb + lipid
Glycoprotein
Carb + protein (look like a Y)
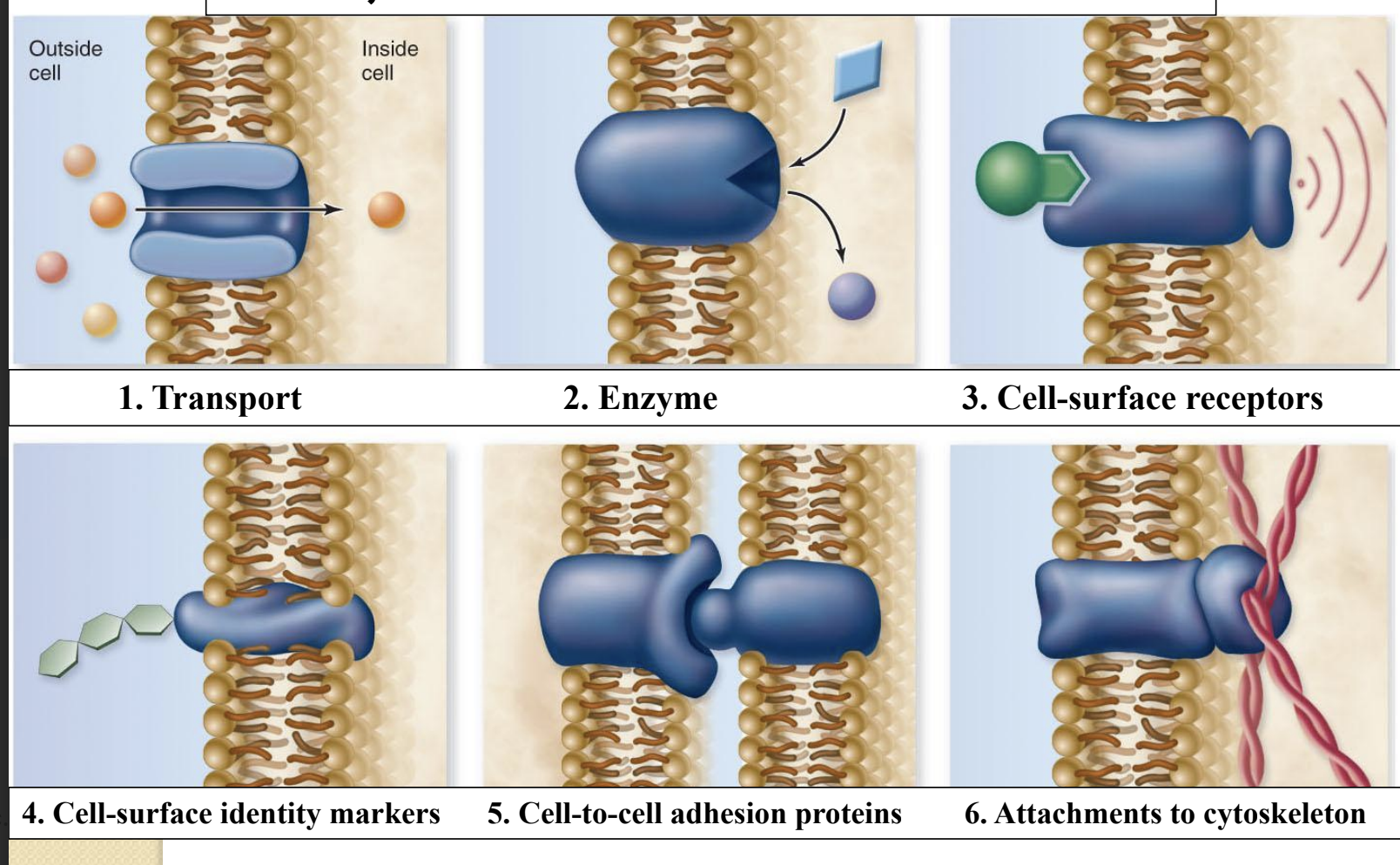
6 Major Functions of Membrane Proteins
Transport
Enzyme
Cell-surface receptors
Cell-surface identity markers
Cell-to-cell adhesion proteins
Attachments to cytoskeleton
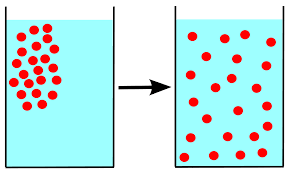
Diffusion
Random movement of particles from high to low concentration
Only occurs if there is a concentration gradient (uneven distribution of solute)
Will continue until equilibrium is reached (even distribution)
A passive process; does not take any added energy to happen
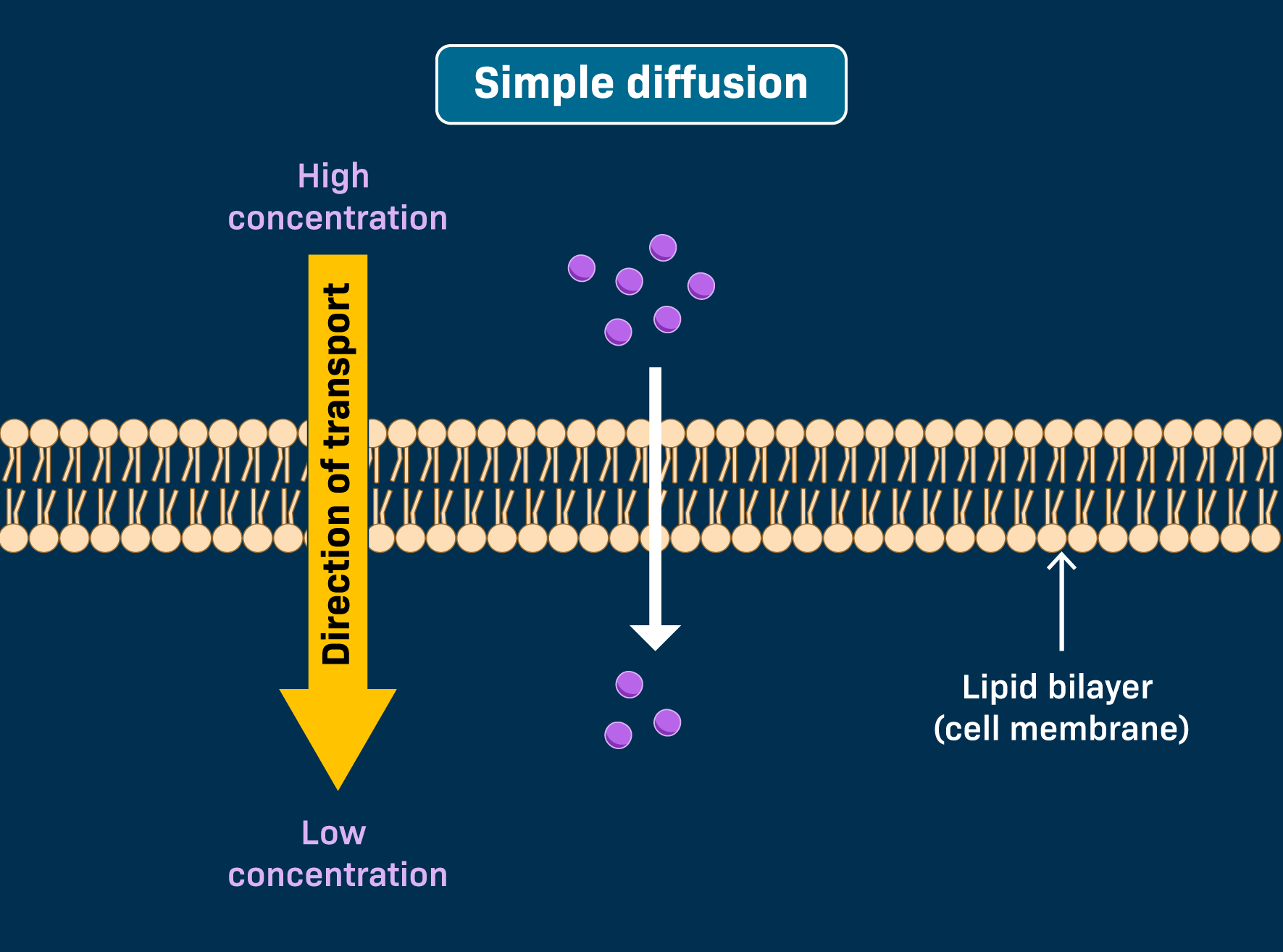
Simple Diffusion
Small, hydrophobic molecules (like H2 and O2) can go right through the phospholipid bilayer
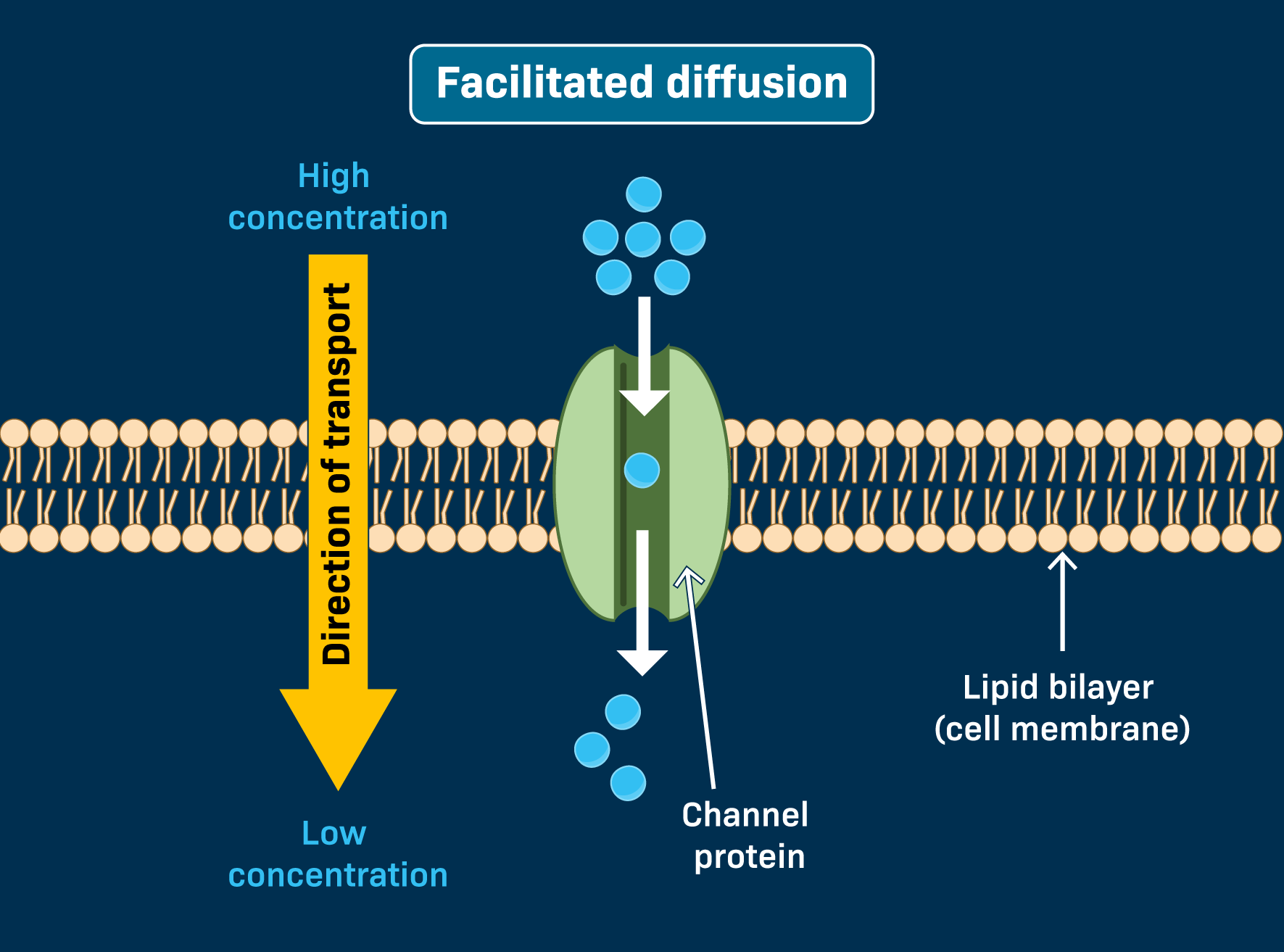
Facilitated Diffusion
Bigger, hydrophilic molecules (like water and sugar) need to use a protein in the membrane to go through
Osmosis
Diffusion of water through a selectively permeable membrane
The membrane will only let water through
Affected by pressure, temp, and solute concentration
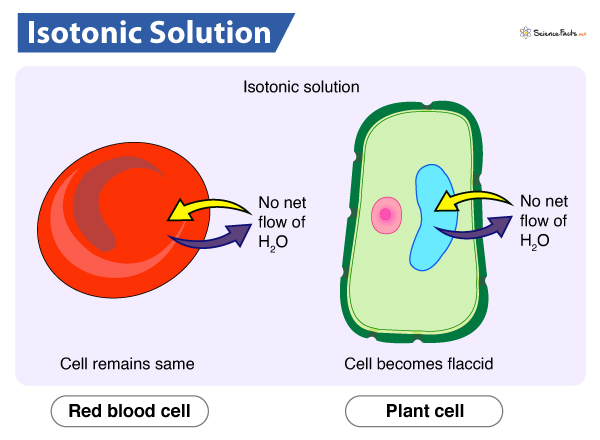
Isotonic Solution
Same concentration of solute inside and outside the cell
No net water movement across the plasma membrane
Animal cells keep their shape
Plant cells wilt/become flaccid
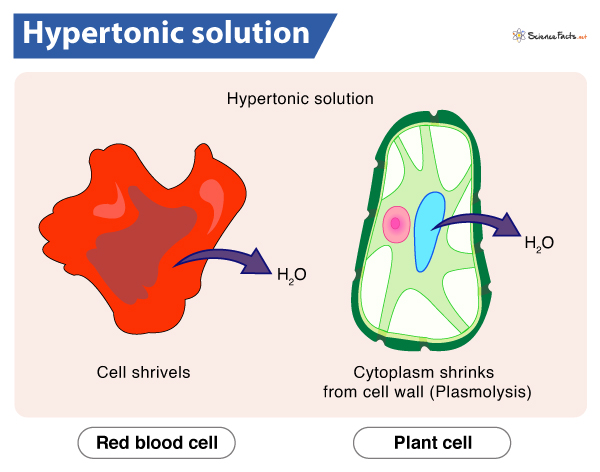
Hypertonic Solution
Higher concentration of solute outside cell than inside
The cell will shrink because water goes out to equalize the concentration
Animal cells shrink and shrivel; still okay
Plant cells tear away from cell walls/become plasmolyzed; not okay
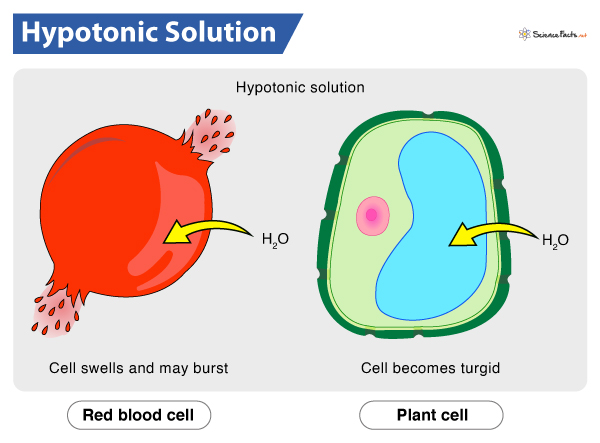
Hypotonic Solution
Lower concentration of solute outside cell than inside
Cell will grow because water goes in to equalize concentration
Animal cells swell and can burst/become lysed; bad
Plant cells are turgid and are happy and normal as the cell wall is tough enough
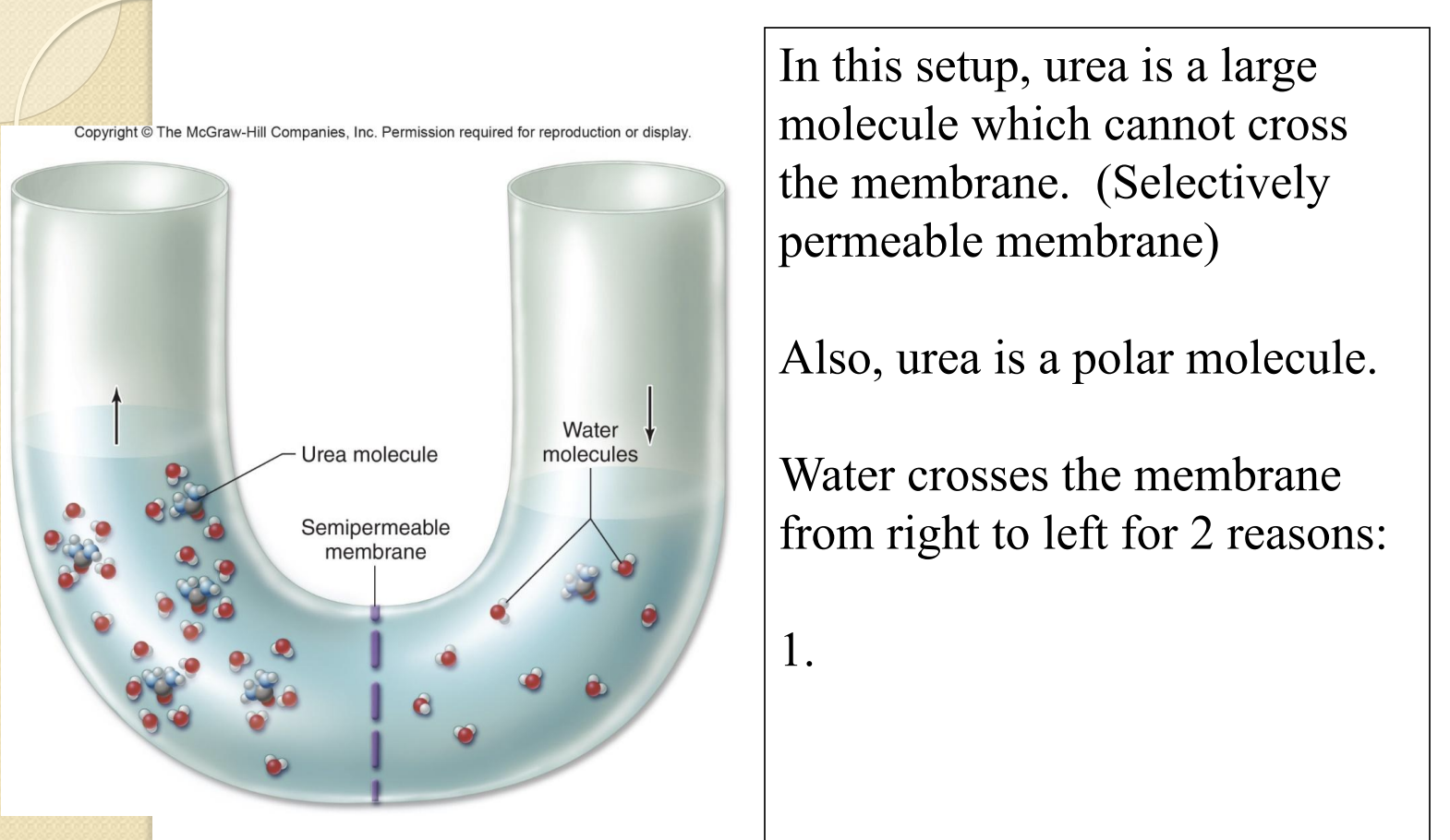
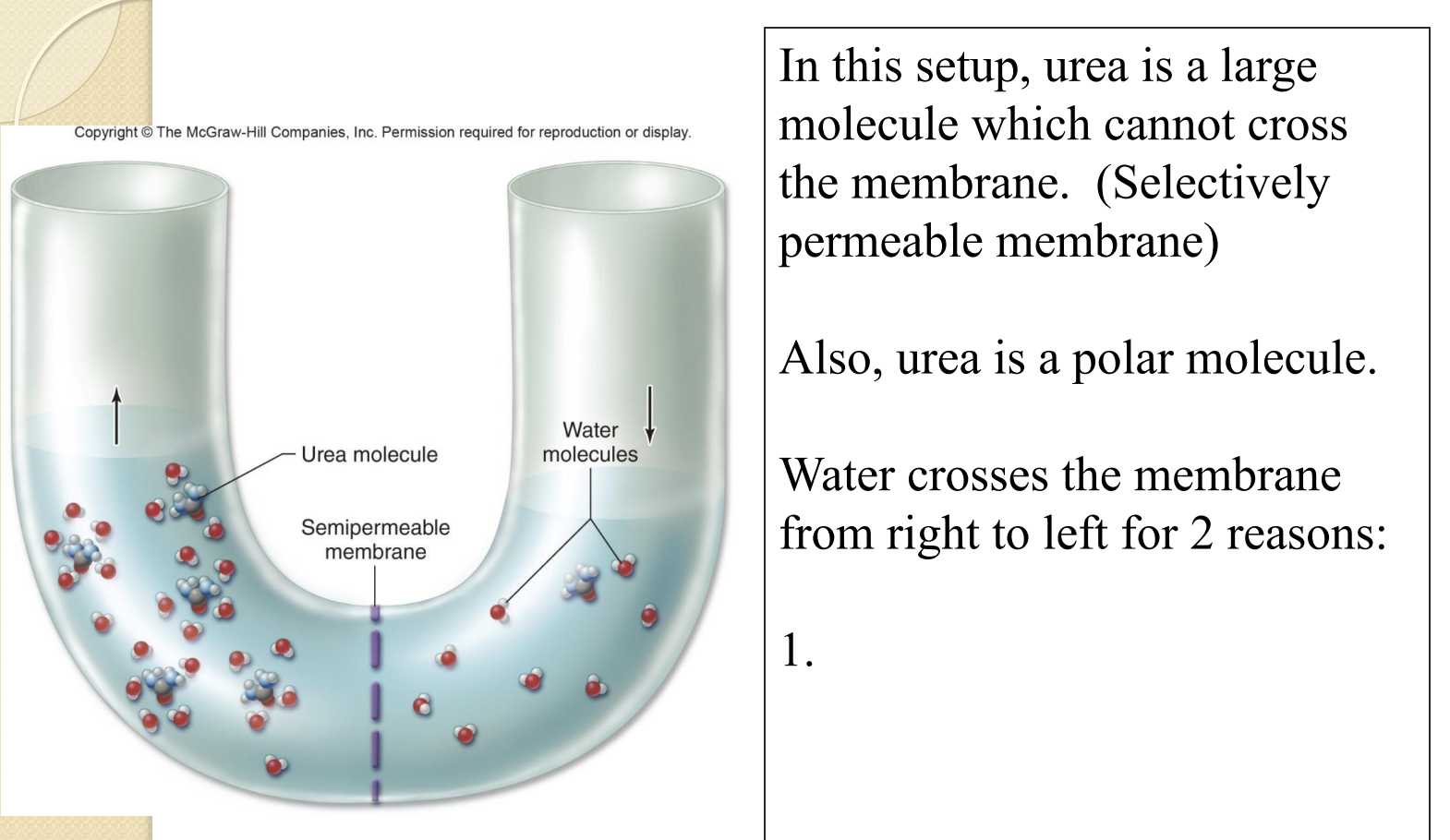
U-Tube and Urea
Urea is polar and water is polar, forming hydration shells as water is attracted to the larger concentration of urea
Equilibrating both sides
Active Transport
Goes against the concentration gradient (unlike simple and facilitated diffusion)
Needs energy (ATP) to pump molecules across the cell membrane
Uses membrane proteins (like facilitated diffusion)
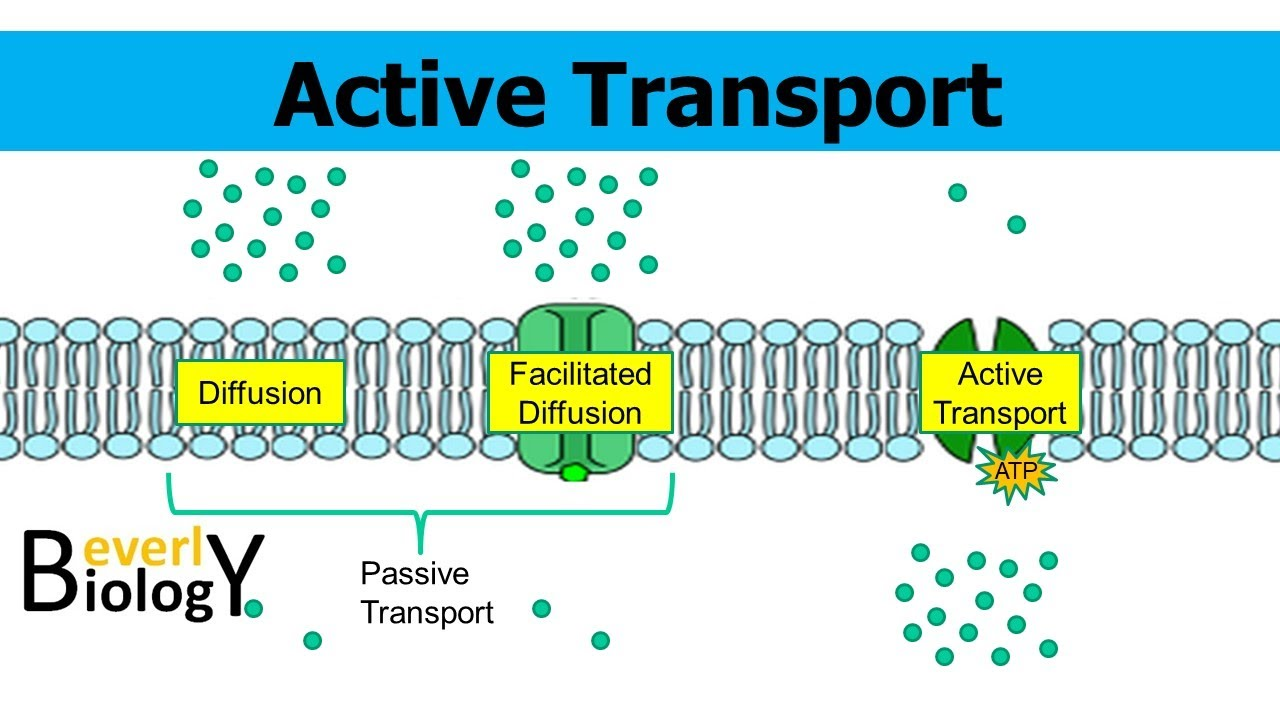
3 Carrier Proteins used in Active Transport
Uniporters
Symporters
Antiporters
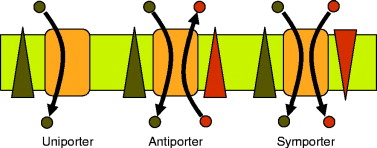
Uniporters
Move one molecule at a time
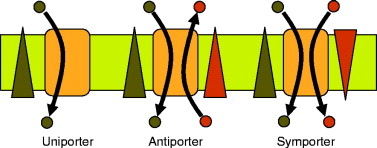
Symporters
Move two molecules in the same direction
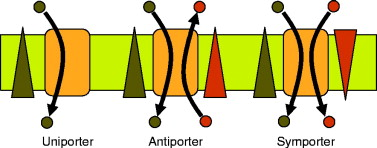
Antiporters
Move two molecules in opposite directions
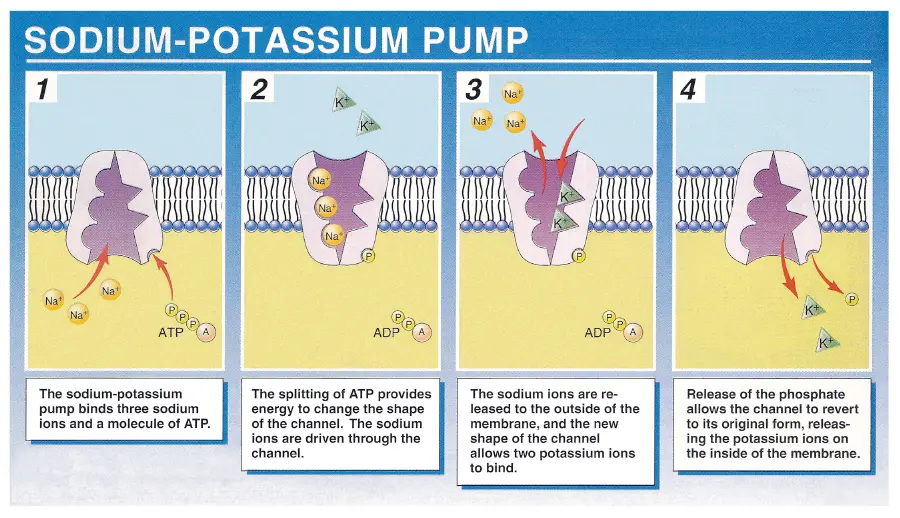
Sodium-Potassium Pump
Uses ATP for active transport
Uses an antiporter to move 3 NA+ out of the cell and 2 K+ into the cell AGAINST their concentration gradient
ATP energy is used to change the conformation of the carrier protein
Affinity of the carrier protein for either NA+ or K+ changes so the ions can be carried across the membrane and then released
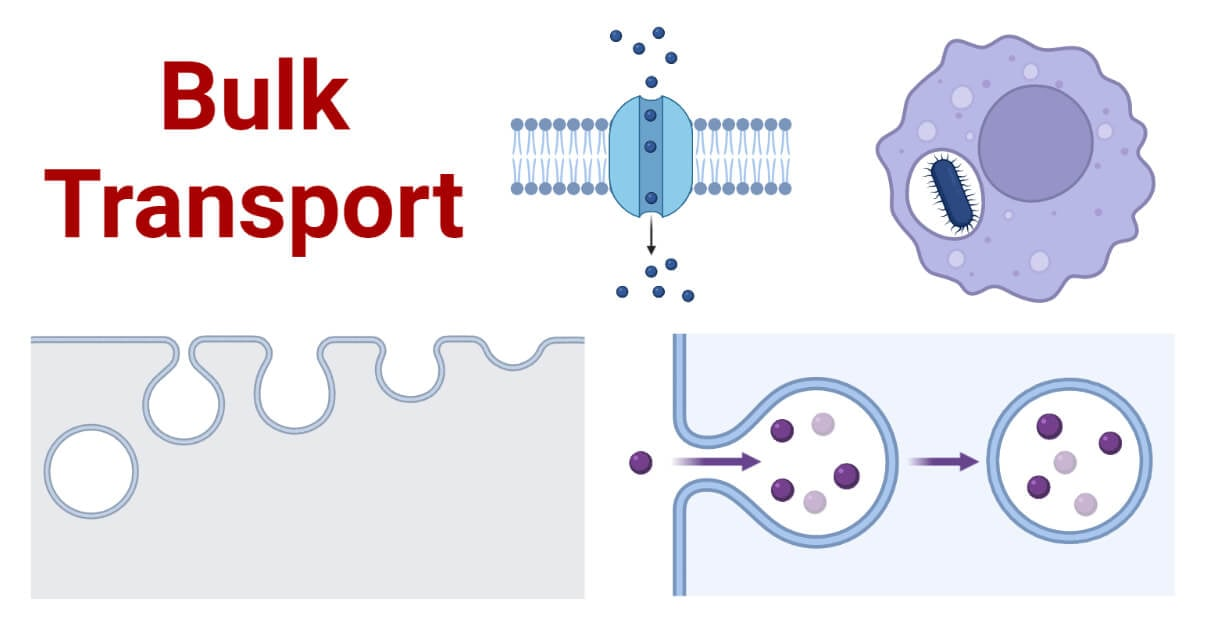
Bulk Transport
Large molecules cross the membrane by vesicles
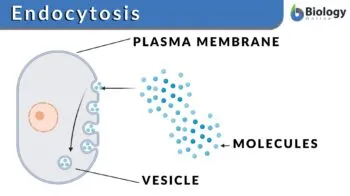
Endocytosis
Movements of substances into the cell
Phagocytosis: Where the cell takes in particles and may fuse with a lysosome for digestion
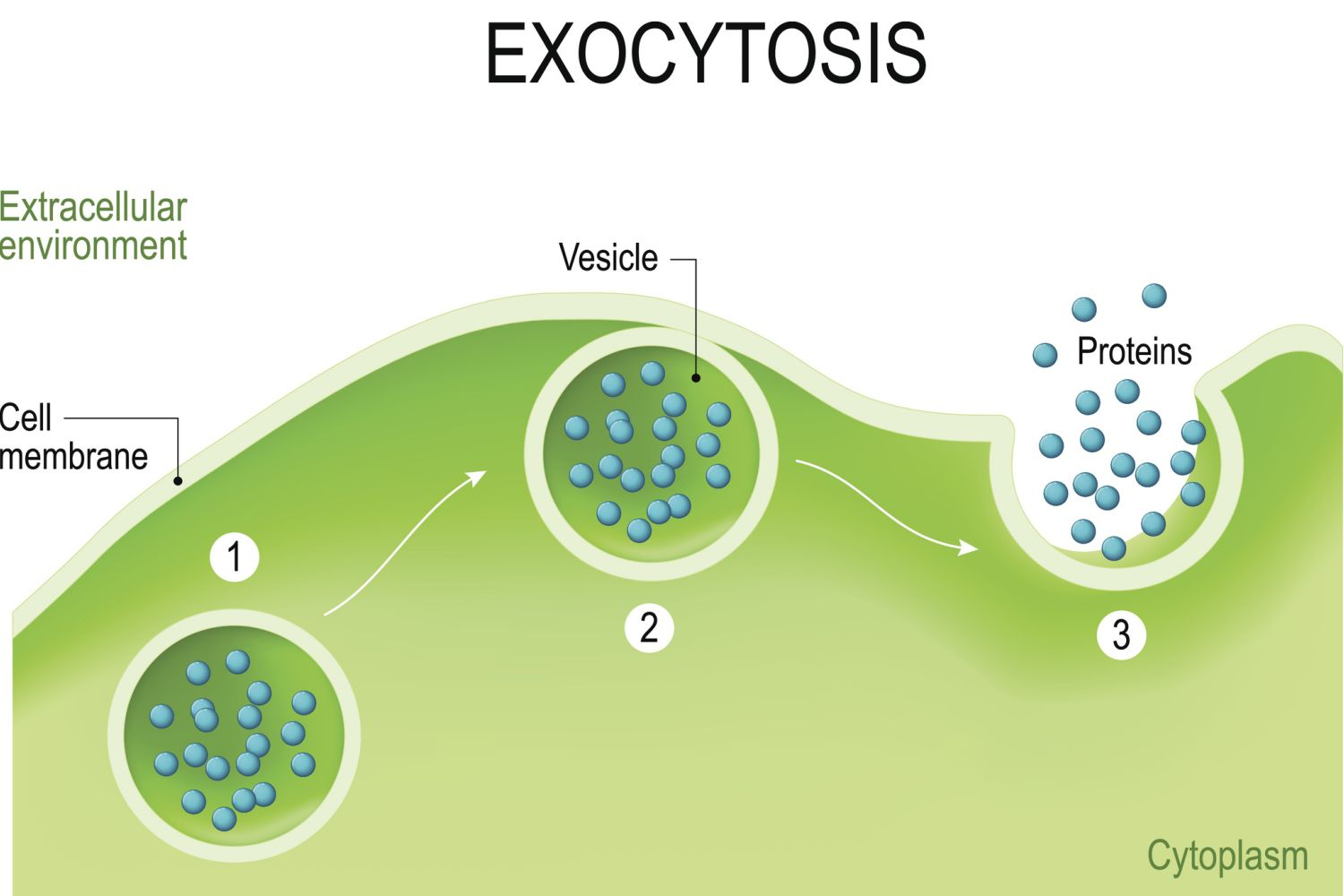
Exocytosis
Movement of materials out of the cell (vesicle fuses with membrane)
Used in plants to export cell wall material
Used in animals to secrete hormones, neurotransmitters, and digestive enzymes
Water Potential (ψw or psi)
A measurement that combines the effects of solute concentration and pressure
Measured in units of pressure like bars or megapascals
Determines the direction of movement of water during osmosis
Water flows from regions of higher water potential to regions of lower water potential
Areas with low water potential (more negative #) tend to pull water and areas with high water potential tend to push water
ψw = 0 for pure water at sea level and room temp
Both pressure and solute concentration affect it
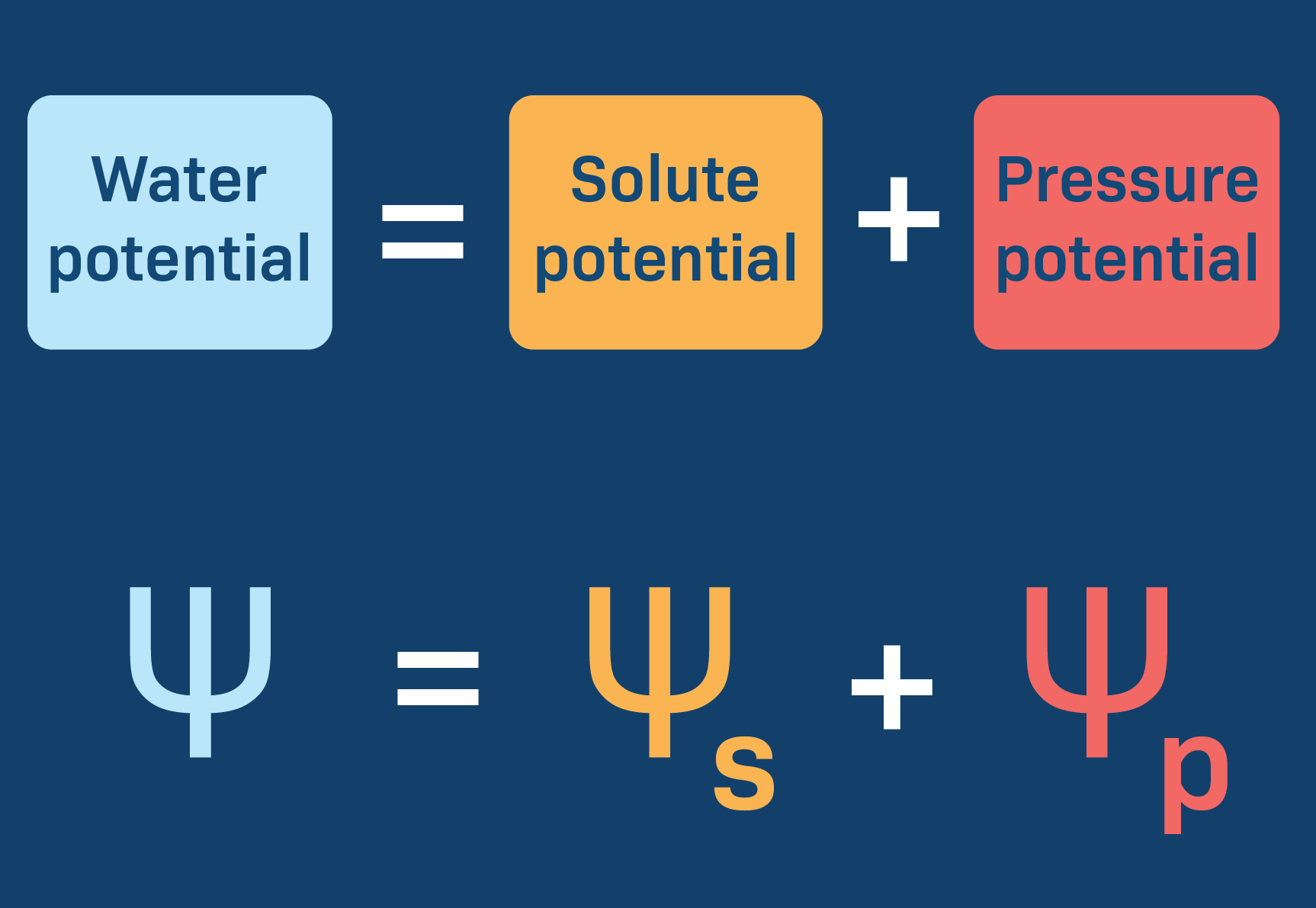
Pressure Potential (ψp)
The physical pressure on a solution
If the cell is an animal cell (no cell wall) and in an open container, ψp = 0
Higher pressure potential from pressure against cell wall = higher pressure potential

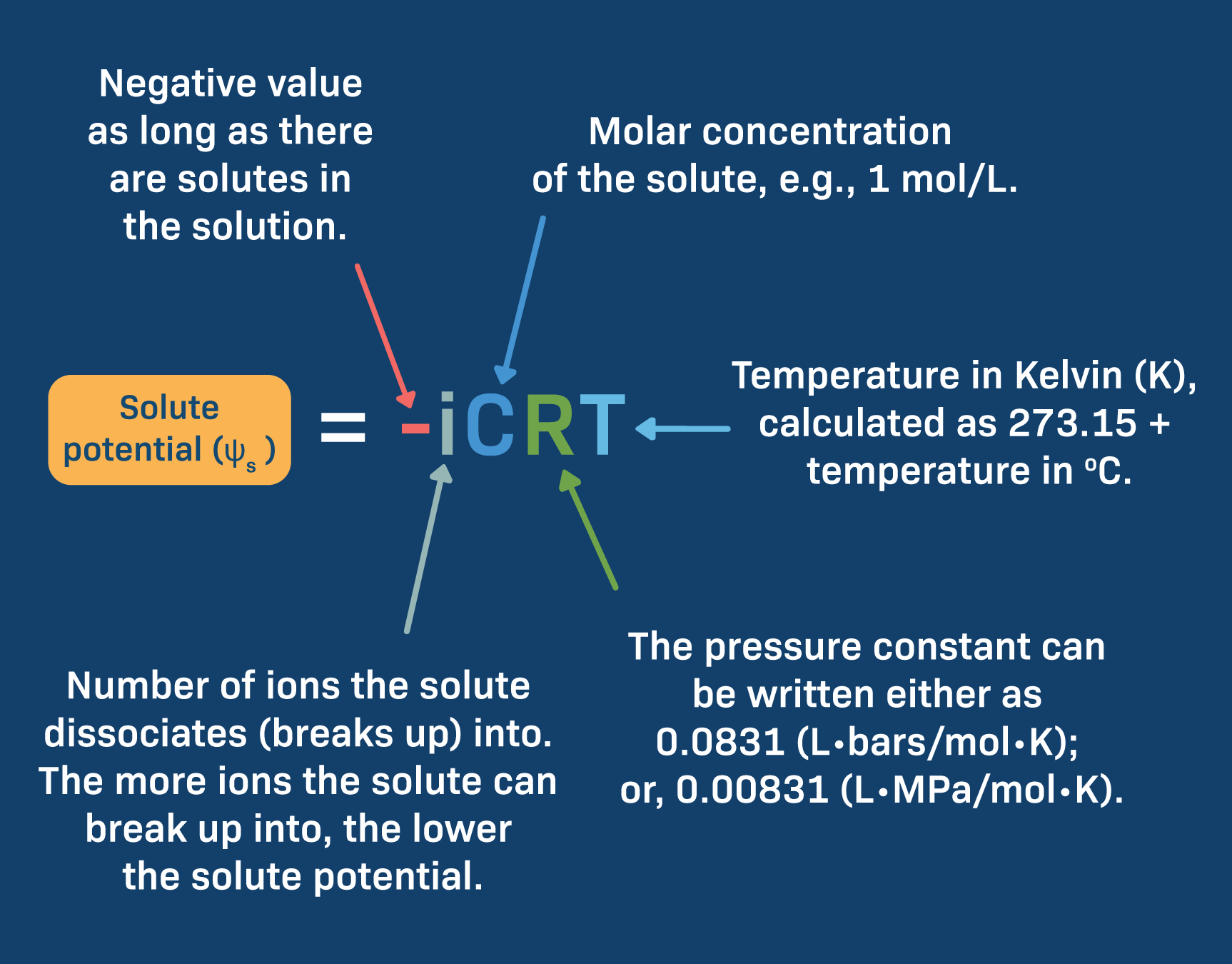
Solute Potential (ψs)
Proportional to the number of types of ions created
Higher solute potential = More negative solute potential
The higher concentration, the more water will be pulled to it
i = Ionization constant
For molecules that do ot break apart or do not ionize, i = 1
Ex. NaCl: i = 2, CaCl2: i = 3
C = concentration (will be given)
R = constant (0.0831 liter bars/mole K)
T = temp in Kelvin (degrees C + 273
The solute potential will always be zero or a negative number