Chem - Ch 6
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
64 Terms
chemical bond
link between atoms that results from the mutual attraction of one atom’s nucleus for another atom’s electrons (and vice versa) resulting in going into a lower energy and more stable
ionic bond
transfer of electrons
covalent bonds
sharing of electrons
metallic bonds
happens in metals when there is only ONE TYPE of electrons (ex. Fe + Fe)
(covalent) polar bond
unequal sharing of electrons, more electronegativity on one side over the other (ex. HCl)
(covalent) non polar bonds
equal sharing of electrons
electronegativity difference - ionic
greater than 1.7 (metal+metal/polyatomic ion)
electronegativity difference - polar covalent
1.7-0.4 (two different nonmetals)
electronegativity difference - nonpolar
0.3 or less (two of the same nonmetals)
endothermic reaction
breaking bonds, must put energy in to break bonds
exothermic reaction
forming bonds, release energy when those bonds are broken
ionic compound
a compound made from positive and negative ions so that the charges are equal. it involves a transfer of electrons
formula unit
the lowest whole number ratio of ion in an ionic compound
metallic ionic bond
lose electrons due to low ionization energy
nonmetallic ionic bond
gain electrons due to high electronegativity
metals and nonmetals go to ___ to have 8 valence electrons
s²p^6
octet rule
the tendency to arrange electrons so each atom has 8
lattice energy
energy released when an ionic compound forms
bond strength relies on
the nuclear charge (number of protons) and the number of valence electrons
sea of electrons theory
the valence Electrons form a free moving sea of electrons that are attracted to multiple positive nuclei
no atoms want the electrons in metallic bonds, so they are
mobile
molecule
smallest quantity of matter that exists by itself and retains the properties of that substance
monatomic molecules
noble gases, He, Ne, Ar, Fe
diatomic molecules
H2, O2, N2, Cl2, Br2, I2, F2 (honcl brif!)
polyatomic molecules
P4, S8, etc.
bond length vs bond energy
bond energy goes up as bond length goes down
shorter bonds are more stable and require more energy to break
longer bonds are less stable and easier to break
multiple bonds result in shorter bond lengths and higher bond energies
sigma bond
a single bond with head on head overlap of orbitals
pi bond
the second bond of a double bond with side to side double overlap of p orbitals (can also be the third bond of a triple bond)
Lewis Dot Diagrams
covalent compounds and polyatomic ions
each atom gets 8 electrons (except H gets 2)
each atom goes for close to the right number of bond
the atom that makes the most bonds goes in the middle (H is always on the outside)
the atom that you have the least of goes in the middle
the least electronegative atom is placed in the middle
then check bonds —> check electrons
charges in Lewis dot diagrams
when atoms get less bonds than atoms normally get = negative charge
when atoms get more bonds than atoms normally get = positive charge
coordinate covalent bond
a bond where two shared electrons in a bond are donated by 1 atom
adding a hydrogen to the single bonded oxygen changes the charge to neutral
H’s go on O’s yeah
Bad Boys of the octet rule
Boron and Beryllium
RHED
bonds + lone pairs
any type of bonds
1 shared pair
linear (1 RHED)
ex. AB
no lone pairs
180 degree angle
linear (2 RHED)
ex. AB2
2 shared pairs, no lone pairs
180 degree angle
bent (3 RHED)
ex. AB2E
2 shared pairs, 1 lone pair
120 degree angle
bent (4 RHED)
ex. AB2E2 (H2O)
2 shared pairs, 1 lone pair
104.5 degree angle
triangular planar (trigonal) (3 RHED)
ex. AB3
3 shared pairs
120 degree angle
triangular pyramidal (pyramid) (4 RHED)
ex. AB3E
3 shared pairs, 1 lone pair
107 degree angle
tetrahedron (4 RHED)
ex. AB4
4 shared pairs
109.5 degree angle
triangular bipyramidal (5 RHED)
ex. AB5
5 shared pairs
120 and 90 degree angles
octahedron (6 RHED)
ex. AB6
6 shared pairs
90 degree angle
intermolecular forces (IMF)
forces that hold diatomic, monatomic molecules, and covalent bonds together
determines the phase of the substance and some properties
intramolecular forces
chemical bonds (ionic, covalent, metallic)
happens within a molecule
always strong
dipole-dipole
forms when electrons are unevenly distributed - polar
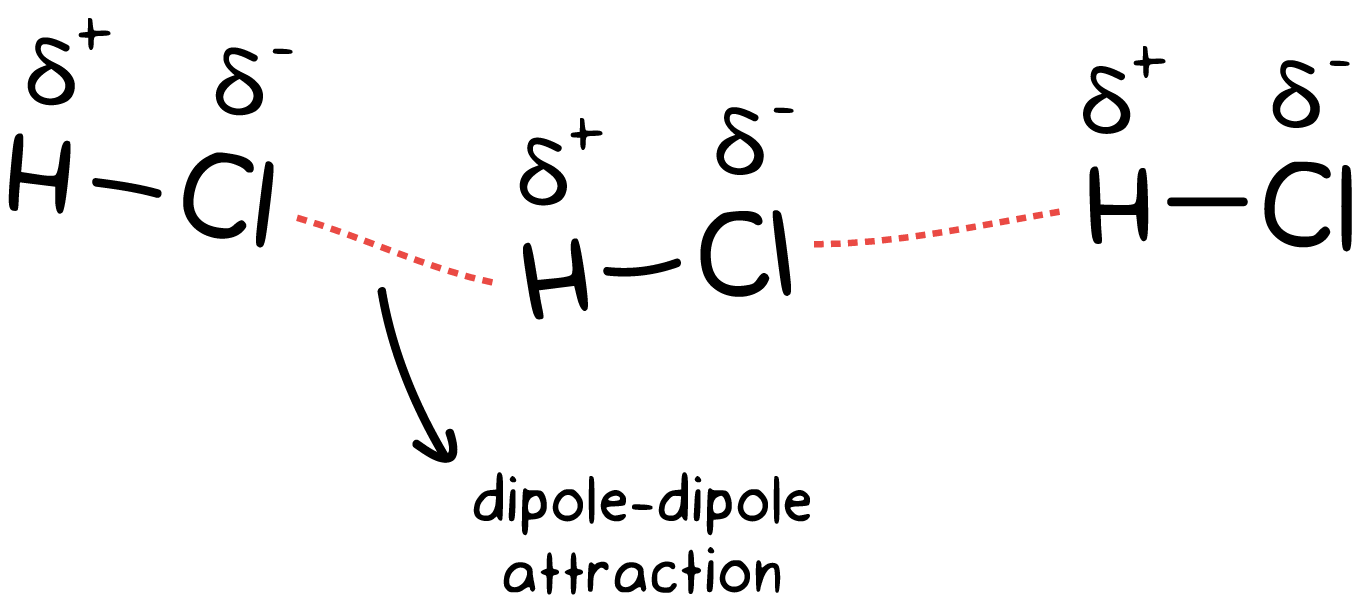
hydrogen bonding
super duper dipole-dipole, only happens with FON
this is what causes freezing water to expand
super high boiling point, heat of vaporization, and melting point
low vapor pressure
Van der Waals forces (London forces)
temporarily induced dipoles caused by the motion of electrons
more electrons = more attraction
nonpolar
bond energy
the energy needed to break a bond - measured in kJ/mole
stronger bond = more stable = needs more energy to break the bond
weaker bond = less stable = little energy to break the bond