Unit 5 - Rare Neurological Diseases & Management
1/158
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
159 Terms
Amyotrophic Lateral Sclerosis (ALS)
Also known as Lou Gehrig disease
Most common motor neuron disease (MND) in adults
Upper motor neuron and lower motor neuron clinical signs and symptoms
ALS
Epidemiology
In 2022, 32,893 cases in the USA
Projected to increase more than 10% in 2030 as population ages 66 years and older
Male is a risk factor (ratio of male to female between 1 to 2).
Mean and median age of onset: 51 and 66 years
Younger age of onset for familial compared to non-familial (sporadic)
ALS
Etiology
Neurodegenerative disorder affecting the corticospinal tract, motor neurons in the motor cortex and brainstem and anterior horn cells in the spinal cord
Familial ALS – 10%
Nonfamilial or sporadic ALS – 90%
Genetic mutation (C9orf72, superoxide dismutase 1 or SOD1)
Excess glutamate
Intraneural protein aggregate
Neuroinflammation
Lack of neurotrophic factor
Environmental factors
Apoptosis and viral infection
ALS
Clinical Presentation — Limb-Onset or Spinal Onset
58 to 82 % of ALS
Starts with a focal, asymmetrical weakness of LE or UE and progress proximally to the trunk
UMN: Spasticity, hyperreflexia, pathologic reflexes, muscle weakness
LMN: Fasciculations, atrophy, muscle weakness, muscle cramps, hyporeflexia, hypotonicity
ALS
Clinical Presentation — Bulbar Onset
25% of ALS
Progresses faster
More common in women, patients with cognitive impairments, older patients
Dysphagia, dysarthria, sialorrhea, pseudobulbar affect
ALS
Clinical Presentation — Respiratory Onset
Less than 3% of the cases
Inspiratory and expiratory muscle weakness
Dyspnea at rest and during exertion, orthopnea, ineffective cough
ALS
Clinical Presentation — Frontotemporal Dementia Onset
presence of the C9 or f72 gene
Cognitive and behavioral impairments
ALS
Pathophysiology
Progressive degeneration and loss of motor neurons
Motor Cortex
Corticospinal tract
Brainstem
Anterior horn cell in the spinal cord
Nonmotor areas
Autonomic nervous system
Basal ganglia
Cerebellum
Frontotemporal
Oculomotor
Sensory Systems
Degeneration>Regeneration
ALS
Diagnosis — Definite ALS
Presence of upper motor neuron and lower motor neuron signs in three anatomical regions
ALS
Diagnosis — Probable ALS
Presence of upper motor neuron and lower motor neuron signs in at least two regions with upper motor neuron sign rostral to lower motor neuron signs
ALS
Diagnosis — Probable ALS, laboratory results supported
Presence of upper motor neuron and lower motor neuron signs in one region with evidence by EMG of lower motor neuron involvement in another region
ALS
Diagnosis — Possible ALS
Presence of upper motor neuron and lower motor neuron signs in one region or upper motor neuron signs in two or three regions, such as monomelic ALS, progressive bulbar palsy, and primary lateral sclerosis
Stages of ALS
Milano-Torino Staging
Based on the ALS Functional Rating Scale (ALSFRS)/ALSFRS- Revised
Four Domains:
Movement
Swallowing
Communication
Breathing
Stages of ALS
Milano-Torino Staging — Stage 0
No loss of independence in any domain
Stages of ALS
Milano-Torino Staging — Stage 1
Loss of independence in one domain
Stages of ALS
Milano-Torino Staging — Stage 2
Loss of independence in two domains
Stages of ALS
Milano-Torino Staging — Stage 3
Loss of independence in three domains
Stages of ALS
Milano-Torino Staging — Stage 4
Loss of independence in four domains
Stages of ALS
Milano-Torino Staging — Stage 5
Death
Stages of ALS
King’s Staging
Based on affected regions:
Upper limb
Lower limb
Bulbar
Stages of ALS
King’s Staging — Stage 1
involvement of first clinical region
Stages of ALS
King’s Staging — Stage 2
involvement of second clinical region
Stages of ALS
King’s Staging — Stage 3
involvement of third clinical region
Stages of ALS
King’s Staging — Stage 4
Nutritional or Respiratory failure
Stages of ALS
King’s Staging — Stage 5
Death
ALS
Prognosis
Average: 27 to 43 months
Five years: 9-40%
Greater than 10 years: 10%
Death usually occurs from respiratory failure
ALS
(+) Prognostic Factors
Younger age
Limb-onset
Less severe involvement at the time of diagnosis
Delayed time between onset and diagnosis
Psychological well-being
ALS
(-) Prognostic Factors
Older age
Bulbar-onset
Respiratory-onset
Frontotemporal dementia-onset
Poor respiratory status
Poor nutritional status
ALS
Medical Management
Disease-Modifying Agents

Symptomatic Management
Sialorrhea and pseudobulbar affect; dysphagia; respiratory impairment; communication impairment; muscle cramp, spasticity, fasciculation and pain; fatigue, and anxiety and depression
ALS
Referral or Consultation
Neurologist
Support Group Liaison
Social Worker
SLP
Registered Dietitian
Occupational Therapist
Physical Therapist
Respiratory Therapist
Mental Health Professional
Nurse
ALS
Evidence-Based Rehabilitative Treatment
Early and early-middle stage
Preventive: Falls, physical inactivity (disuse atrophy)
Restorative/ Maintenance: Physical function
Compensatory: Assistive device, orthosis
ALS
Evidence-Based Rehabilitative Treatment
Late-middle to late stage
Compensatory: caregiver education, adaptive equipment and home modifications to maximize function and quality of life, DMEs (hospital bed, transfer equipment e.g. mechanical lift, wheelchair)
Preventive/ Restorative: secondary complications from immobility (pressure ulcer, VTE, limited passive range of motion e.g. adhesive capsulitis, pulmonary complications)
ALS
Physical Therapy Examination
Communication and Cognition
Cardiovascular and Pulmonary System
Cardiovascular – blood pressure, heart rate, RPE
Pulmonary – breathing pattern, respiratory rate, lung auscultation, oxygen saturation, cough strength, Dyspnea ALS-15, Motor Neuron Disease Dyspnea Scale, Fatigue Severity Scale
Integumentary
Skin integrity
Edema
Musculoskeletal
ROM
MMT
Pain
Neuromuscular
Cranial nerve integrity
Postural alignment, control and balance
Gait
Sensation
Tone and reflexes
Outcome measures to check for risk for falls
Functional
ALSFRS-R
Psychosocial
ALS Depression Inventory
Environmental
Quality of life
ALS Assessment Questionnaire
ALS Quality of life short form
ALS
Physical Therapy Interventions
Cervical Muscle Weakness
Head falls forward, overstretched posterior muscles, tight anterior muscles
Difficulty in eating, drinking, swallowing
Difficulty seeing forward and communicating
Acute cervical pain, Chronic cervical syndrome
Accompanied by shoulder girdle and thoracic extensor weakness
ALS
Physical Therapy Interventions
FOR Cervical Muscle Weakness
Collars
soft collar – mild weakness
semirigid or rigid collar – moderate to severe weakness
Head up collar
Cervical-thoracic orthosis
Frequent rest periods
Supportive seating
Head and neck support for high back chairs, reclining or tilt-in space wheelchair
ALS
Physical Therapy Interventions
FOR Dysarthria and dysphagia
Referral to a SLP and nutritionist
Head and trunk control
Sitting position
ALS
Physical Therapy Interventions
Pain
Shoulder, neck, back pain
20% incidence of adhesive capsulitis
Shoulder subluxation
ALS
Physical Therapy Interventions
FOR Pain
Modalities
ROM exercises
Passive stretching
Joint mobilizations
Proper positioning in bed, wheelchair, chair
Joint support and protection
Intraarticular analgesics or anti-inflammatory injection
ALS
Physical Therapy Interventions
FOR UE weakness
Referral to Occupational Therapist for ADL
Adaptive equipment
Splinting such as wrist and hand splint
ALS
Physical Therapy Interventions
Respiratory muscle weakness
Breathing or coughing
ALS
Physical Therapy Interventions
FOR Respiratory muscle weakness
Referral to a respiratory therapist, physician
Energy conservation techniques
Breathing exercises and positioning
Airway clearance techniques
ALS
Physical Therapy Interventions
FOR LE weakness and gait impairments
Orthosis
§ AFO for weak ankle dorsiflexion
Assistive device
§ Wheeled walker
ALS
Physical Therapy Interventions
FOR Decreased mobility
Bed mobility
Transfers
Ambulation and stair climbing
Wheelchair mobility
DMEs:
Hospital bed, transfer board, hydraulic or mechanical lifts, power mobility device
ALS
Physical Therapy Interventions
FOR Muscle cramps and spasticity
Massage, stretching and passive ROM
Postural and positioning technique
ALS
Physical Therapy Interventions
Psychosocial issues
Anxiety, depression
ALS
Physical Therapy Interventions
FOR Psychosocial issues
Referral to an appropriate healthcare provider.
ALS
Exercise Prescription
To prevent disuse atrophy (cardiovascular deconditioning and muscle weakness) caused by
physical inactivityPrescribing exercise for people with ALS
ROM and stretching
Aerobic/endurance and strengthening exercises for early or early-middle stage and more slowly progressive disease
Resistance exercise for unaffected muscle and possibly affected muscle which can tolerate resistance (greater than a grade 3) using a low to moderate load and intensity
Aerobic exercise e.g. walking, cycling, swimming) at submaximal levels (50 to 65% of heart rate reserve)
ALS
Overwork Weakness
Overuse damage
Caused by excessive exercise or activities to the point of extreme fatigue
High repetition
High intensity
Heavy resistance
Eccentric exercise
Signs and symptoms
Inability to perform ADL post-exercise because of exhaustion or pain
Decreased muscle force that gradually recovers
Increased or excessive muscle cramping, soreness, fatigue, or fasciculations
Research Study: Is exercise safe and effective for ALS?
Intervention: 24 sessions over 12 weeks (2 sessions per week), 50-60 minutes
Aerobic training (20-30 minutes) recumbent cycling 40-60% of HRR
Flexibility (10 minutes) achieved by stretching and PROM
Strength training (20 minutes) functional exercise using body weight 8-12 reps, 1-2 sets on large muscle groups of trunk, UE and LE, e.g planks
Outcomes: Improved maximum expiratory pressure, 2-minute walk test, ALSFRS-R, SF-36 (physical functioning, energy fatigue and wellbeing) in the aerobic-strength group. No adverse events
Research Study: Is exercise safe and effective for ALS?
Interventions (3 days per week for 24 weeks)
Resistance exercise group: concentric upper limbs and hip flexion using cuff weights, knee flexion and extension using weight bench, 2 sets of 8 reps at 40% (weeks 0-2), 50% (weeks 3-4) and 70% of 1RM (weeks 5 to 24)
Endurance exercise group: minicycles 10 minutes UE, 10 minutes LE, 5 minutes warm-up and cool down 40-70% target HR, RPE somewhat hard to hard
Stretching and ROM (SROM) exercise group: 4 reps 30 seconds: shoulder flexion, stretching of triceps, hands and wrist, quadriceps, hamstrings, gastrocnemius
Outcomes:
SROM, endurance and stretching tolerable for 12 and 24 weeks, with endurance less well-tolerated than SROM at both time-points
Compliance was highest for both SROM and resistance than endurance
Most common adverse effect was falling but less in endurance and resistance exercise groups.
No increase in fatigue, pain or muscle cramps
Electrodiagnostic Testing
Nerve Conduction Studies
Evaluate motor/sensory nerve function:
Involvement of PNS
Location of PNS involvement
Intensity of PNS involvement
Systemic/localized involvement
Motor/Sensory involvements
Equipment needed
Surface electrodes
Adjustable stimulation trigger
Computer/computer software
Speakers
Electrodiagnostic Testing
Influential Factors
Age – Normal nerve conduction velocity values are not achieved until age 7 (mature at 18)
UE/LE injury
UE NCV is faster
Height/limb length
Longer limbs have slower conduction velocities
Extremity temperature
Colder limbs conduct signals more slowly
Anomalies with innervation patterns
Electrodiagnostic Testing
Who can administer NCS?
Depends on the state
Most states allow PTs to administer
Some states allow Chiropractors to administer
States may require specific certifications before administration
Electrodiagnostic Testing
Motor Nerve Conduction Study
Compound Muscle Action Potential (CMAP)
Simultaneous depolarization of all motor units under a recording electrode
Procedure
1. Surface electrodes on distal musculature supplied by the nerve
Active electrode: close to motor point
Reference electrode: over tendon (somewhere not excitable)
Ground: over bony prominence
2. Nerve stimulated at various sites within the path
3. Second stimulation site for consistency
Electrodiagnostic Testing
Orthodromic
When the max intensity stimulus is given, like a lightning bolt feeling, the motor signal is conducted along the motor fibers in its normal direction toward the muscle, which causes a contraction.
This contraction is seen on the nerve conduction screen as an M-wave and measures the distal latency from the stimulus to the muscle
Electrodiagnostic Testing
Antidromic
At the same time the signal's conducted orthodromically, it is also conducted away from its normal direction to the anterior horn cells of the spinal cord
The spinal cord then sends an action potential back to the muscle orthodromically and you see another small contraction.
This smaller contraction is called the F-wave and measures distal latency in both proximal and distal directions
Electrodiagnostic Testing
Calculation of NCS
Two sites are stimulated
Nerve conduction values are given:
Linear measurement between the 2 sites
(R = reference electrode, 1 = site 1 and 2 = site 2)
The following calculation can be made:
MNCV (m/sec) = Dist. between prox/distal sites(m) / prox latency – distal latency (msec)
Compare MNCV to a chart with normal values
Increased NCV = Slow/latency
Electrodiagnostic Testing
H-Reflex
Another long loop reflex that can be taken similar to the F-wave.
Monosynaptic stretch reflex
Acquired by stimulating the Ia afferent (as opposed to the motor nerve that is stimulated to obtain the F-wave)
Electrodiagnostic Testing
Sensory Nerve Conduction Study
Similar to Motor NCS
Differences:
SNAP (sensory nerve action potential)
No NMJ shows a direct response
Single point rather than 2
Smaller amplitudes
Electrodiagnostic Testing
Clinical Electromyography (EMG)
To assess the motor point more specifically
Uses a needle electrode – “Current density”
Eliminates extraneous noise of overlying tissues
More Uncomfortable
Identifies a more clear understanding of location of injury and which specific motor units are injured
Electrodiagnostic Testing
Equipment for EMG
Same as for Nerve Conduction Studies
No electrical stimulation involved
Needles for specificity
Electrodiagnostic Testing
EMG Outcomes
Provides information regarding:
Innervation integrity
Evidence of motor unit recovery
Neuropathic or myopathic findings
Localization of injury to specific patterns
ie. Injury consistent with anterior horn cell (ALS, polio), nerve root (tumor, HNP), plexus (stretch/compression), or NMJ (MG) disorder
Electrodiagnostic Testing
EMG Procedure
1. Insertion
2. Rest
3. Minimal Activation
4. Maximal Activation
Electrodiagnostic Testing
Insertion
Normal, increased, sustained, decreased or absent
Stability of the muscle membrane
NORMAL = brief electrical activity then activity ceases
ABNORMAL= Prolonged or Absent
Electrodiagnostic Testing
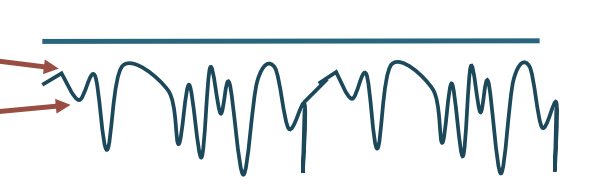
Rest
When needle ceases to move
Electrical silence

MEPs (miniature end plate potentials)
When tip of needle is near NMJ

End-plate Spikes
Short duration
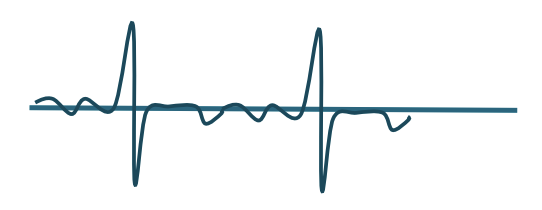
Begin with upward (negative) wave
Electrodiagnostic Testing
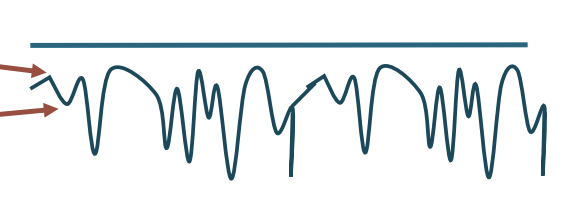
Rest (abnormal output)
Positive Sharp waves (downward spike)
Sound similar to a dull thud
Abnormally sensitive muscle membrane
Fibrillations
Similar to Positive Sharp waves
Very short duration
Sounds like rain on a tin roof
Fasiculations
Popping sound
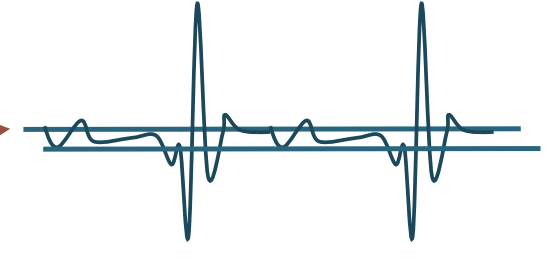
Electrodiagnostic Testing
Minimal Activation
Assesses motor units with voluntary contraction (MUP)
NORMAL = Biphasic or triphasic
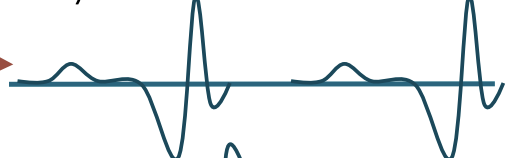
ABNORMAL = polyphasic (5 + phases)
Low amplitude, long duration
Early re-innervation

Large amplitudes
Chronic neuropathies
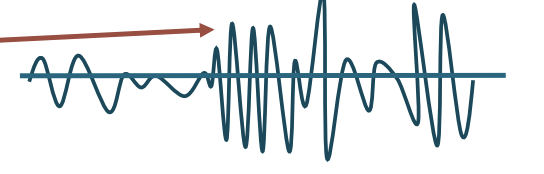
Low amplitude, short duration
Myopathic disease
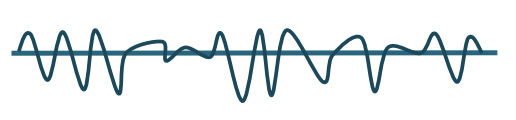
Electrodiagnostic Testing
Maximal Activation
To observe the orderly recruitment of MUPs
NORMAL = “interference pattern”
ABNORMAL =
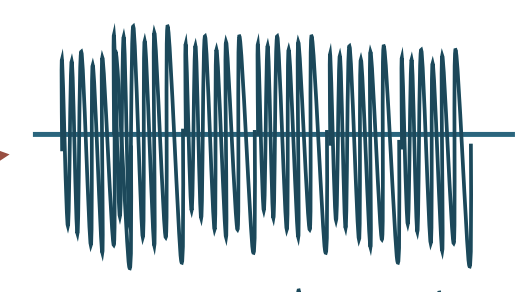
Neuropathic recruitment pattern
Decreased recruitment due to denervation
Myopathic recruitment pattern
Small amplitude with little to no effort
Decreased activation

Brain Tumors
1.5% of all cancer diagnoses
Primary versus secondary brain tumors
Malignant versus nonmalignant
Brain Tumors
Incidence and Etiology
In 2019, nonmalignant tumors new cases > malignant tumors
Overall prevalence as of December 31, 2019, was 1,323,121 cases, 14.6% were malignant.
In children (ages 0–14 years), majority was malignant
Adults ages 40+ years, Glioblastoma was most prevalent primary malignant brain and other CNS tumor
Astrocytoma was the most prevalent of all malignant brain and other CNS tumors
Meningioma had the highest overall prevalence (1/3 of all brain tumors), the majority of which were non-malignant (99%)
95% are nonhereditary; exposure to ionizing radiation is a risk factor
Classification of Tumors
Gliomas
the most common malignant tumor in adults and children; arise from supportive tissues of the brain
Classification of Tumors
Gliomas — Astrocytomas
From astrocytes (star-shaped glial cells); frontal lobe in adults, cerebellum in children
Classification of Tumors
Gliomas — Glioblastomas
Highly malignant grade IV astrocytoma; more common in older adults, males>females; medical prognosis is poor
Classification of Tumors
Gliomas — Oligodendrogliomas
Slow-growing but progressive; frontal and temporal lobes; 4th to 6th decade of life, male>female
Classification of Tumors
Gliomas — Ependymomas
From ependymal cells; most common site is 4th ventricle, primarily treated with surgical resection and shunt
Classification of Tumors
Gliomas — Medulloblastomas
Malignant embryonal tumors; cerebellum and 4th ventricle; children and adult <45, males>females
Classification of Tumors
Meningiomas
slow-growing; dura and arachnoid membrane; increases with age; female>males
Classification of Tumors
Pituitary adenomas
benign epithelial tumors; frequently encroach the optic chiasm; hypersecretion or hyposecretion of hormones; more common in older people; females>males
Classification of Tumors
Schwannomas
Schwann cells; cranial or spinal nerves; example: acoustic neuroma
Classification of Tumors
Primary CNS lymphomas
rare; from lymphatic system; male>female; 6-8th decades; poor prognosis;
Classification of Tumors
Secondary Brain Tumors: Metastatic Brain Tumors
Tumor originate outside of CNS and spread to the brain through arterial circulation
80% in cerebral hemisphere
20% posterior fossa
Origin: (1) lungs, (2) breast, (3) skin, (4) gastrointestinal tract, (5) kidney
Brain Tumors
Signs and Symptoms
Headaches § 50 % of cases
Interrupts with sleep or worse when waking up, improves during the day
Elicited by postural changes, coughing or exercise
Location is determined by tumor’s location
Seizures
Frequent symptom, 20-50% of adults with brain tumor
First seizure during adulthood is suggestive of seizure
> 50% of gliomas, 11% of brain metastases
Altered mental status
Common in front lobe tumors and elevated ICP
Papilledema
Swelling of optic nerve
Temporary visual loss with position changes
More common in children
Vomiting and dizziness
Tumor in posterior fossa
Elevated ICP
Specific signs and symptoms
Depends on the functional areas of the brain
Brain Tumors
Diagnosis
MRI
Diffusion-Weighted Imaging (DWI)
Magnetic Resonance Spectroscopy
Functional MRI
Perfusion Weighted Imaging
Molecular Imaging
Stereotactic Biopsy
Molecular diagnosis
Brain Tumors
Medical and Surgical Treatment
Traditional Surgery
Craniotomy
Partial and complete tumor resections
Brain Tumors
Medical and Surgical Treatment
Chemotherapy
Intravenously via peripherally inserted central catheter (PICC) or tunneled access central catheter (TACC); disrupt ability of tumor cells to replicate
Brain Tumors
Medical and Surgical Treatment
Radiation therapy
Tumors too large or inaccessible for surgical resection; residual tumor cells; limited by maximum lifetime dosage of radiation
Brain Tumors
Medical and Surgical Treatment
Stereotactic Radiosurgery
High dose of ionizing radiation on a precisely defined volume of tissue; disrupt tumor DNA; for lesions less than 3cm; Gamma Knife, linear accelerators, cyberknife
Brain Tumors
Medical and Surgical Treatment
Hormonal Therapy
Pituitary tumors
Brain Tumors
Medical and Surgical Treatment
Immunotherapy
Biotherapy; most infrequently used and least-proven; improve immune system
Brain Tumors
Intracranial Surgery Considerations
Observe for intracranial bleeding or seizure for at least 24 hours
Monitor blood pressure, deep vein thrombosis (1/3 of patients) or pulmonary embolism, CSF leakage, wound infection, periocular edema, pneumocystis carinii, meningitis
CSF leaks: Avoid Valsalva, coughing, sneezing or blowing of nose
If bone flap is removed, avoid positioning on the operative side
Brain Tumors
Increased Intracranial Pressure (ICP)
Major complication of intracranial surgery
Resulting from cerebral edema or bleeding
Positioning: Avoid lowering of the head. Elevate head 20-30 degrees in bed.
Symptoms: decreased level of consciousness, headache, visual and speech disturbances, muscle weakness, pupil changes, seizures, vomiting, respiratory changes
Emergency treatment for ICP >20mmHg: mannitol, hyperventilation and corticosteroid
Surgical intervention: shunt
Brain Tumors
Side Effects and Considerations for Chemotherapy & Radiation Treatment
Hair loss
Fatigue
Nausea
Skin burns or irritation
Difficulty eating or digesting food
Anorexia
Dry, sore mouth
Low blood count (Anemia, infection, hemorrhage)
Vomiting
Diarrhea or constipation
Cognitive or personality changes
Brain Tumors
Steroid Effects
Reduce Cerebral Edema From Surgery or Radiation
Side-effects Of Long-term Use:
Proximal Weakness
Behavioral Changes
Osteoporosis
Increased Appetite
Bloating
Hypertension
Brain Tumors
Cancer-Related Fatigue
Causes:
myelosuppression, anorexia, pain, sleep deprivation, side effect of cancer treatment
Screen:
European Organization for Research and Treatment of Cancer – Quality of Lift (30 core questionnaire)
Assessment:
Piper Fatigue Scale-revised, Functional Assessment of Chronic Illness Therapy – Fatigue, Patient Reported Outcome Measurement Information System (PROMIS) Fatigue SF.
Intervention:
Structured progressive exercise program: 50 to 70% of heart rate reserve, RPE of 11 to 14, avoid isometrics and vigorous resistive exercises
Brain Tumors
Physical Therapy Evaluation — Karnofsky performance scale
Evaluate impact of tumor or treatment
Functional outcomes similar to stroke or TBI
Functional outcome scales
Determine the meaning of quality of life from a patient’s perspective
Brain Tumors
Physical Therapy Intervention
Physical conditioning
Functional Training
Range of motion
Lymphedema management
Pain management
Recommendation of appropriate assistive device, adaptative equipment and home modification
Caregiver education and training
Postpolio Syndrome (PPS)
Postpolio muscular atrophy
New neuromuscular symptoms after recovery from acute poliomyelitis
Review of Acute Poliomyelitis:
caused by the polio virus (enterovirus) affected mainly children under 3 years old
Inflammation of the meninges and anterior horn cell resulting to loss of spinal and bulbar motor neuron.
Led to asymmetric, flaccid paralysis with LE more affected than UE, and bulbar weakness in 10 to 15% of cases.
Polio vaccines: Salk (1950s) and Sabin (1960s) vaccine
Postpolio Syndrome
Etiology
Unknown
Most common theory: increase size of innervation ratio, wherein a small axon innervates many muscle fibers which leads to an overworked motor unit. Giant motor units cannot sustain the metabolic demands of their sprouts.
Muscle fiber type changed from Type II (fast twitch) to Type I (slow twitch)
Postpolio Syndrome
Pathogenesis
Muscle denervation
Attrition of motor neuron that can no longer support axonal sprouts
Postpolio Syndrome
Clinical Presentation
New muscular weakness
Muscle fasciculation, cramps, atrophy and elevation of muscle enzyme in the blood
Fatigue
Major complaint
Most common and debilitating
Generalized, focal or central
Pain
Parts of body previously affected by polio, cramping of lower extremity, aching of neck and shoulders, associated with mechanical stress, related to physical activity
Loss of function
Bulbar
Respiratory and swallowing
Cold intolerance