Chapter 10/11? - Intermolecular Forces
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
52 Terms
Intramolecular Bonding:
Within” the molecule. aka Ionic, Covalent, Metallic, Network Covalent bonding
Molecules are formed by transferring/sharing electrons between the atoms.
Intermolecular Forces:
Forces that occur between molecules.
Ion-Dipole Forces
Dipole-Dipole Forces
Hydrogen bonding
London dispersion forces
Intramolecular bonds are significantly stronger than Intermolecular forces.
Hydrogen Bonding in Water:
Blue dotted lines are the intermolecular forces between the water molecules.
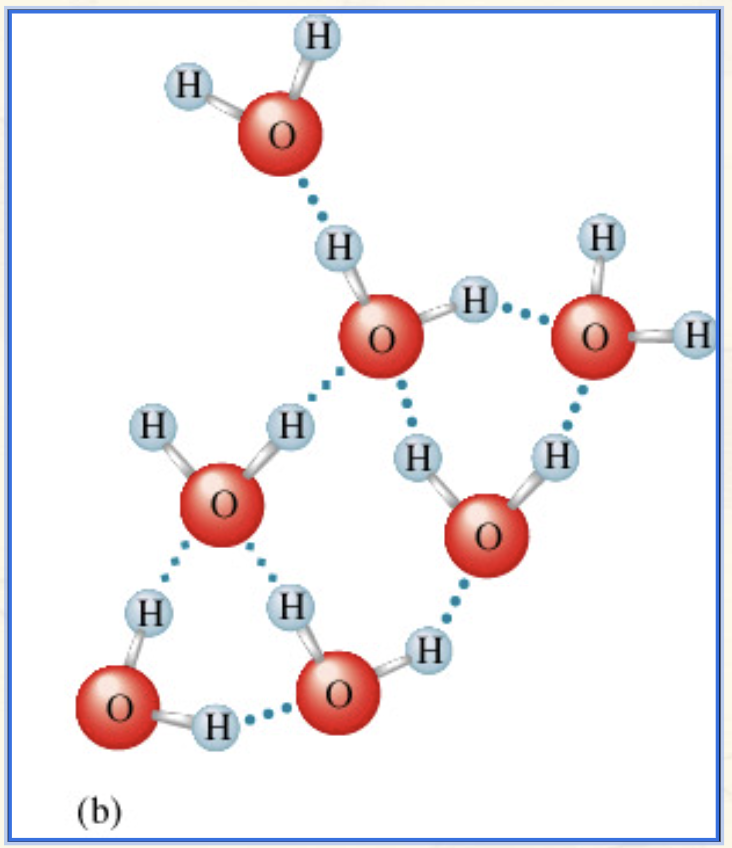
Which are stronger, intramolecular bonds or intermolecular forces?
How do you know?
Intramolecular bonds are stronger because it would take a lot more energy to overcome covalent bonds and break apart the molecule than to overcome intermolecular forces in between the atoms (to make it become a liquid or gas).
Phase Changes:
When a substance changes from solid to liquid to gas, the molecules bonds remain intact.
The changes in state are due to changes in the forces between the molecules rather than in those within the molecules.
Schematic Representations of the Three States of Matter
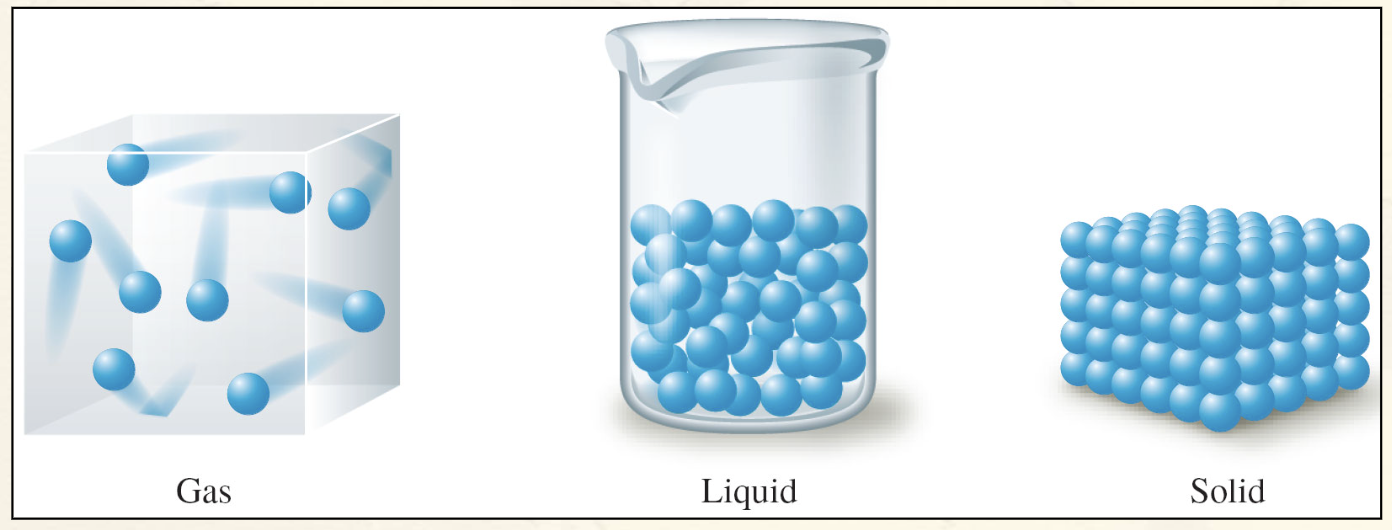
Phase Changes:
Solid to Liquid
As energy is added, the motions of the molecules increase, and they eventually achieve the greater movement and disorder, characteristic of a liquid.
Liquid to Gas
As more energy is added, the gaseous state is eventually reached, with the individual molecules far apart and interacting relatively little.
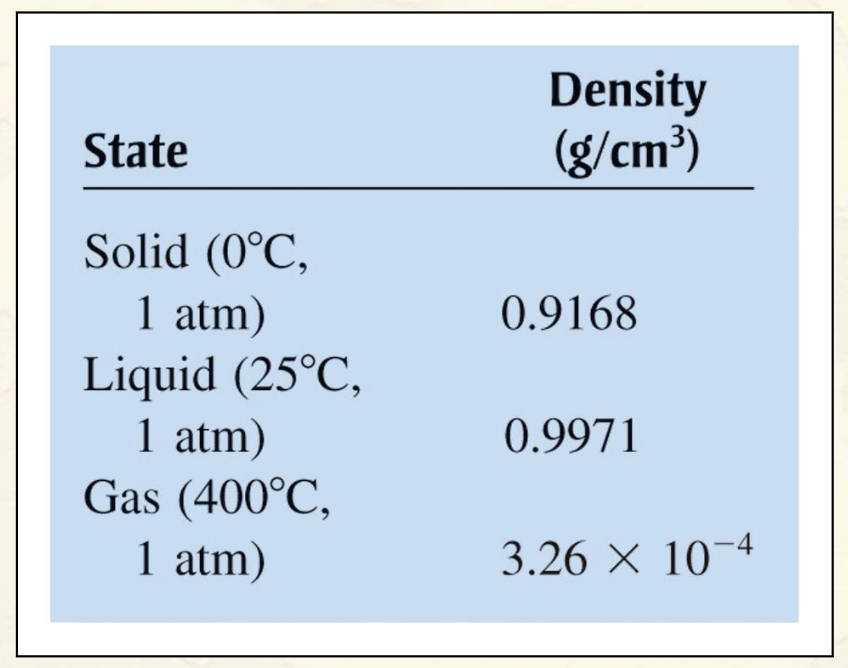
Densities of the Three States of Water
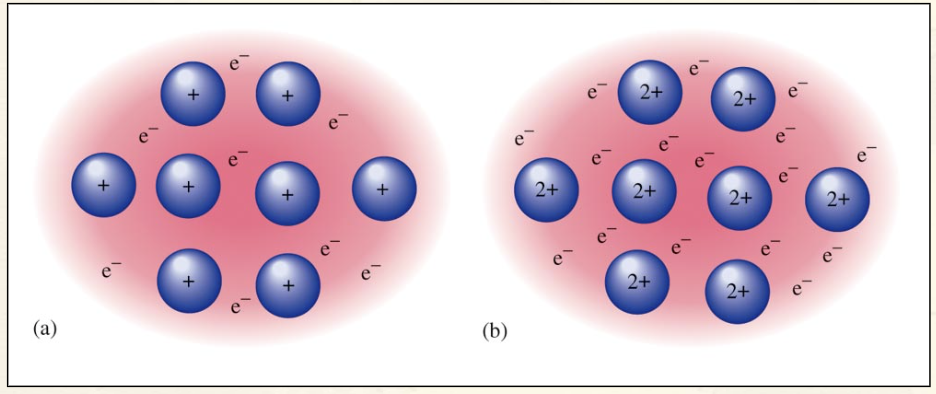
Ion Dipole Forces:
Ion-dipole forces are stronger than dipole-dipole interactions because the charge of any ion (whole number) is much greater than the charge of a dipole (fractional)
The strength of ion-dipole interactions is dependent on the charge and the distance (Coulomb’s again!) The ion-dipole force is proportional to ionic charge and dipole moment.
Dipole-Dipole Forces:
Dipole moment – molecules with polar bonds often behave in an electric field as if they had a center of positive charge and a center of negative charge.
Molecules with dipole moments can attract each other electrostatically. They line up so that the positive and negative ends are close to each other.
Only about 1% as strong as intramolecular forces such as metallic, covalent, or ionic bonds.
Hydrogen Bonding:
Unusually strong dipole-dipole forces
Hydrogen is bound to a highly electronegative atom – nitrogen, oxygen, or fluorine. The H on one molecule is attracted to the lone pair of electrons (of N, O, F) on a neighboring molecule
London Dispersion Forces:
Occur in all molecules, but is the ONLY IMF present in nonpolar molecules.
More significant in large atoms/molecules with big electron clouds
Instantaneous dipole is a short lived event that occurs randomly in a given atom’s electron cloud which induces a similar dipole to a neighboring atom, creating a force of attraction
Strength of LDFs are influenced by the polarizability of the electron cloud. More electrons = more polarizable = stronger LDFs
Longer chain hydrocarbons have stronger LDFs than shorter chains due to bigger and more polarizable electron clouds. Branched hydrocarbons have weaker LDFs due to less surface area of the molecule
Melting and Boiling Points:
In general, the stronger the intermolecular forces, the higher the melting and boiling points
Which molecule is capable of forming stronger intermolecular forces?
N2 or H2O
H2O has the stronger intermolecular forces because it exhibits hydrogen bonding and LDFs, whereas N2 only exhibits London dispersion forces.
Draw two Lewis structures for the formula C2H6O and compare the boiling points of the two molecules.
One Lewis structure could be ethanol and one Lewis structure could be dimethyl ether. Ethanol will have a higher boiling point than dimethyl ether because ethanol exhibits hydrogen bonding and LDFs. Dimethyl ether exhibits dipole-dipole interactions and LDFs. Hydrogen bonding is an especially strong type of dipole-dipole interaction and will thus have a higher boiling point as ethanol.
Which gas would behave more ideally at the same conditions of P and T?
CO or N2
N2 would behave more ideally because it is nonpolar and only exhibits London dispersion forces, therefore the intermolecular forces between N2 molecules are weak (and thus the collisions will be more “elastic”). CO exhibits dipole-dipole interactions and LDFs.
Liquids:
Low compressibility, lack of rigidity, and high density compared with gases.
Surface tension
Surface Tension:
resistance of a liquid to an increase in its surface area:
surface molecules are pulled toward the interior
PE is increased for molecules at the surface
interior molecules are attracted in all directions
Liquids with large intermolecular forces tend to have high surface tensions.
Capillary action
spontaneous rising of a liquid in a narrow tube:
Cohesive forces – intermolecular forces among the molecules of the liquid.
Adhesive forces – forces between the liquid molecules and their container.
Concave Meniscus Formed by Polar Water:
Which force dominates alongside the glass tube – cohesive or adhesive forces?
adhesive forces
Viscosity:
measure of a liquid’s resistance to flow:
Liquids with large intermolecular forces or molecular complexity tend to be highly viscous.
Two types of solids:
Amorphous Solids:
Disorder in the structures
Glass
Crystalline Solids:
Ordered Structures
Unit Cells
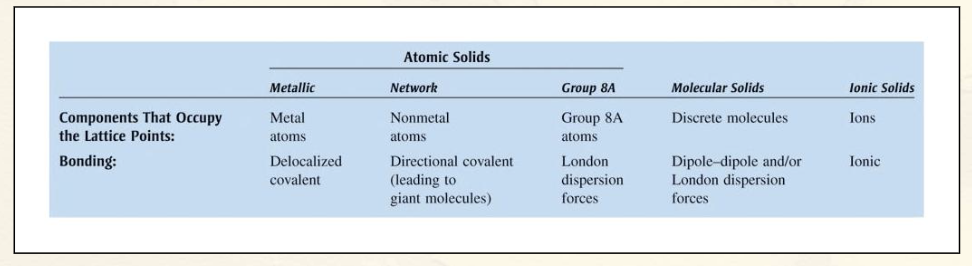
Types of Crystalline Solids:
Ionic Solids – ions at the points of the lattice that describes the structure of the solid.
Molecular Solids – discrete covalently bonded molecules at each of its lattice points.
Atomic Solids – atoms at the lattice points that describe the structure of the solid.
Classification of Solids (Picture):
Closest Packing Model:
Closest Packing:
Assumes that metal atoms are uniform, hard spheres.
Spheres are packed in layers.
The two bonding models for metals:
Electron Sea Model
Band Model (MO Model)
The electron sea model:
A regular array of cations in a “sea” of mobile valence electrons.
The two types of metal alloys:
Substitutional Alloy – some of the host metal atoms are replaced by other metal atoms of similar size.
Interstitial Alloy – some of the holes in the closest packed metal structure are occupied by small atoms.
Brass made up of copper and zinc is what kind of alloy?
Substitutional - copper and zinc are relatively similar sizes
Steel is made up of iron and carbon, what type of alloy is it?
Interstitial - iron and carbon are very different sizes
Vapor Pressure:
Pressure of the vapor present at equilibrium, know as the pressure water exerts on air.
The system is at equilibrium when no net change occurs in the amount of liquid or vapor because the two opposite processes exactly balance each other.
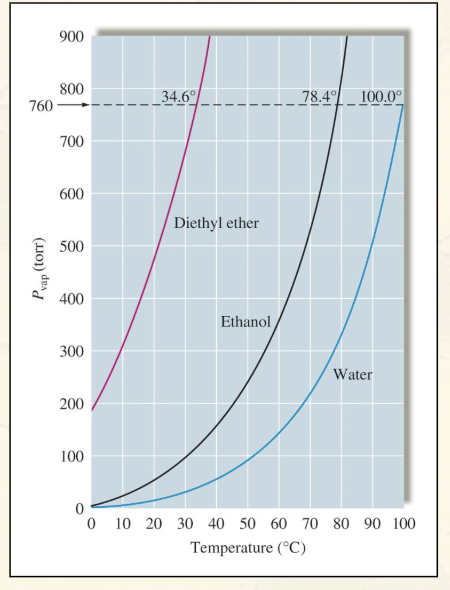
What is the vapor pressure of water at 100°C? How do you know?
1 atm
The vapor pressure of water at 100C is 1 atm. You know this because atmospheric pressure is 1 atm and this is the temperature at which we observe water to boil.
Liquids in which the intermolecular forces are strong have what kind of vapor pressures?
Relatively low vapor pressures
What is the ONLY way to significantly increase vapor pressure?
With temperature.
Low vapor pressure means what kind of IMFs and boiling point?
Strong IMFs and a higher boiling point
Volatile is
the tendency of a substance to vaporize
Vapor Pressure vs. Temperature (Picture):
What three ways can you represent the phases of a substance as a function of temperature and pressure?
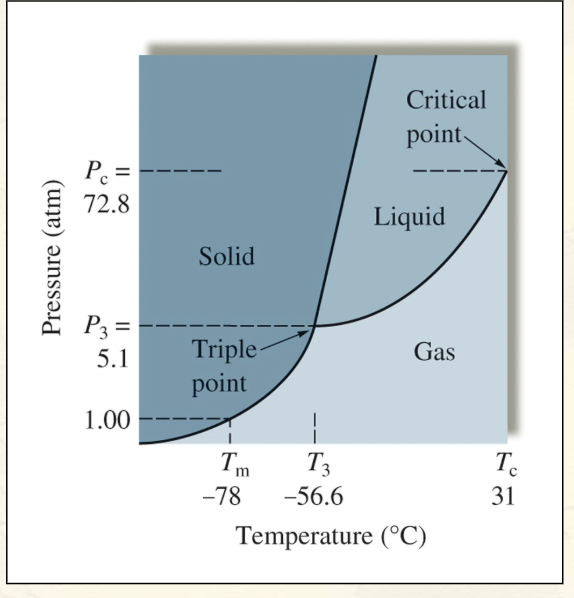
Triple point - conditions of temp and pressure that allow all 3 phases of a substance to exist simultaneously
Critical point - the temp and pressure at which the vapor and the liquid phases become indistinguishable
Phase equilibrium lines - show what conditions of temp and pressure allow for that specific state of matter
The Phase Diagram for Carbon Dioxide (Picture, be able to identify triple point, critical point, and phases of matter areas):
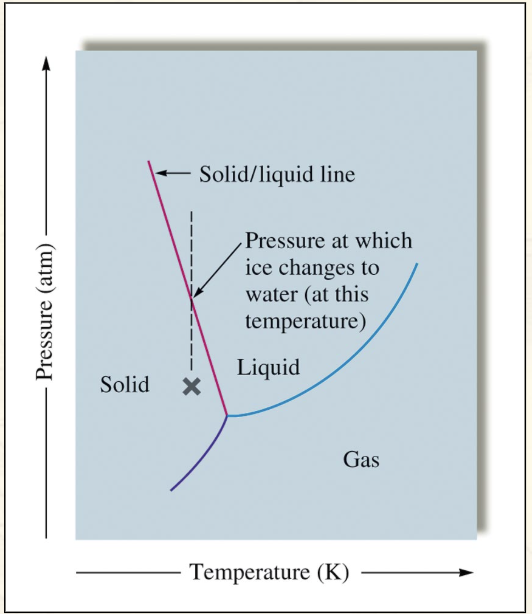
The only phase diagram that has a negative slope is:
Water
As intermolecular forces increase, what happens to each of the following? Why?
Boiling point
Viscosity
Surface tension
Enthalpy of fusion
Freezing point
Vapor pressure
Heat of vaporization
Boiling point: Increases because more thermal energy is required to overcome the stronger attractive forces and transition from the liquid to the gas phase.
Viscosity: Increases because the stronger forces lead to greater resistance to flow, as molecules are more strongly attracted to each other and less able to move past one another.
Surface tension: Increases because stronger attractive forces between molecules at the surface pull them more strongly toward the bulk of the liquid, requiring more energy to increase the surface area.
Enthalpy of fusion (Heat of fusion): Increases because more energy is needed to break the strong intermolecular forces during the transition from a solid to a liquid (melting process).
Freezing point (Melting point): Increases because stronger IMFs hold the molecules more tightly in the solid crystal structure, requiring a higher temperature to provide the energy needed to overcome these forces and melt.
Vapor pressure: Decreases because the stronger forces make it more difficult for molecules to escape from the liquid phase into the vapor phase (evaporation rate is lower), resulting in fewer gas-phase molecules above the liquid at equilibrium.
Heat of vaporization (Enthalpy of vaporization): Increases because more energy is necessary to overcome the attractive forces and convert the liquid into a gas
The 4 types of solids:
Molecular Solids
All non-metal atoms held by weak IMFs
Ionic Solids
Cations and Anions held by ionic bonds
Metallic Solids
All metal atoms held by electron sea
Network Covalent Solids (i.e., diamonds)
Covalent bonds but in networks (large structures)
The 2 Structures of Solids:
Crystalline
Neat, ordered, with consistent
bond lengths
Amorphous
Disorganized, almost random alignment of atoms
Molecular
Held together by weaker van der Waals forces
Lower melting points
Think ice!
Ionic
Held by ionic bonds
Brittle
Higher melting points
Conductive when dissolved
Metallic
Held by electron sea
Even Higher melting points
Always conductive
Network Covalent
Held by covalent bonds
Strong / harder materials
Highest melting points!
Network Covalent Solids All Properties:
Non metals and semi metals
Insoluble in water
Have extremely high melting points
Metal atoms are held together by covalent bonds
Bad conductors of electricity
Metallic Solids All properties:
Formed of Metals Only
Insoluble in water'
Have very high melting points
Metal atoms are held together by metallic bonds
Conduct electricity in all forms
Ionic Solids all properties:
Metals and Nonmetals
soluble in water
high melting points similar to metals
formed of ions that are bonded together by ionic bonds
conduct electricity when dissolved or when molten
Molecular solids all properties:
Contain only nonmetals
some are soluble in water
low melting points
formed of molecules that are connected by intermolecular forces
do not conduct electricity
What type of solids will each of the following form?
NaCl
Aluminum
Copper
Brass (copper-zinc)
Silicon
Graphite
Copper Sulfide
C2H6O2
Ice water
Which will have the lowest melting point? Highest?
Ionic
Metallic
Metallic
Metallic - Substitutional
Network Covalent
Network Covalent
Ionic
Molecular
Molecular
Water
Graphite