2.6 Unit #2 Lesson#6 Heating and Cooling Curves
0.0(0)
Card Sorting
1/11
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
1
New cards
What is the first part in the chart?
Matter
2
New cards
What is matter divided into the chart?
1.(Pure) Substances
2. A mixture of Substances.
2. A mixture of Substances.
3
New cards
1. (Pure) Substances can be divided into?
Elements and Compounds.
4
New cards
2. Mixture of Substances can be divided into?
Homogenous and a Heterogenous mixtures.
5
New cards
Pure Substances
Matter that has uniform or defined shape.
EX: Water is H20
EX: Water is H20
6
New cards
Elements
Is the simplest form of matter that has an unique set of properties.
-Cannot be broken Down
-Cannot be broken Down
7
New cards
Compunds
2 or more elements chemically combined in a fixed proportion
-Can Only be broken down by Chemical Reaction.
-Can Only be broken down by Chemical Reaction.
8
New cards
2. Mixture of Substances
Matter that has 2 or more substances combined and the composition of each substance can vary
9
New cards
homogenous mixture (solution)
A mixture that has uniform and is the same throughout.
-One phase and components are visually.
-One phase and components are visually.
10
New cards
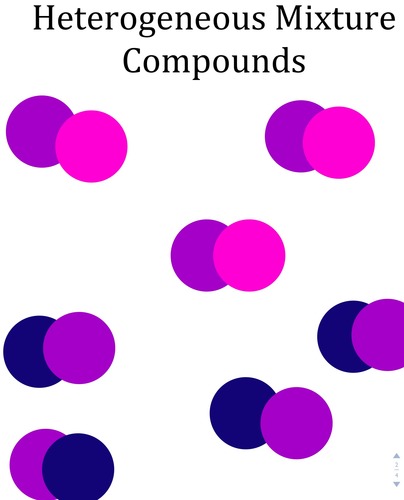
Heterogenous mixture
A mixture that is not uniform (the same) throughout
-Components are not evenly distributed
-Separated Phases.
-Components are not evenly distributed
-Separated Phases.
11
New cards
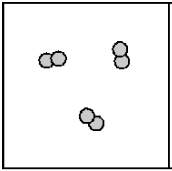
Compound(Particle Diagram)
2 molecules onw is colored the other is not.
12
New cards
Element(Particle Diagram)
2 molecules stuck together but same color