Recrystallisation - purifying an impure solid
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Last updated 12:10 PM on 12/16/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
1
New cards
Method of recrystallisation:
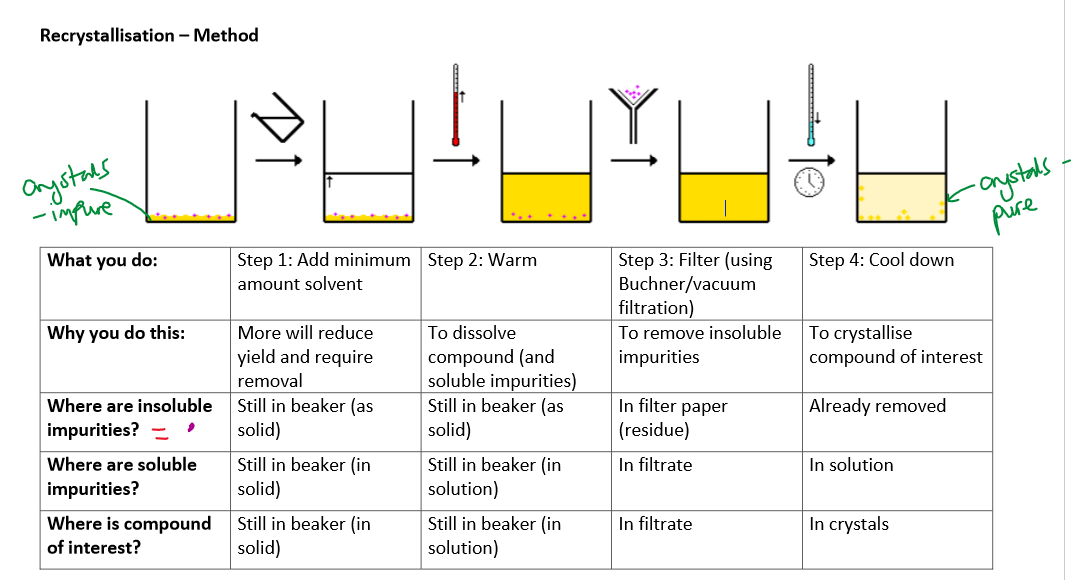
2
New cards
How can the crystals be recovered?
the crystals of your compound can then be recovered by filtering once more (and washing with cold solvent to remove further filtrate if necessary)
the soluble impurities will pass through the filter by the crystals won’t
(Note that any solvent used will used to wash with must be cold to avoid redissolving the crystals and reducing the yield)
3
New cards
What is the advantage of recrystallisation?
get a purer product
4
New cards
What is the disadvantage of recrystallisation?
reduces % yield - due to transfer losses (lots of steps)