C3: Rates of Reaction
1/10
Earn XP
Description and Tags
grade 9
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
successful collisions
in order to have successful collisions, particles must:
a) collide
b) have sufficient energy — more than activation energy
c) correct orientation
Temperature
Increasing temperature increases the average kinetic energy of the particles. This increases the proportion of particles with the required activation energy. This increases the frequency of successful collisions — increases rate of reaction .
Concentration
Increasing the concentration increases the number of particles in a given volume. This increases the likelihood of collisions, which increases the frequency of successful collisions.
Surface Area
A larger surface area means more sites where collisions can happen, increasing likelihood of collisions, increasing frequency of successful collisions.
High Yield/Optimal
High yield: producing a large amount of product relative to the amount of reactant used.
Optimal: conditions producing highest yield at minimum cost
Rate of reaction
change in concentration of reactants/products over unit time
Measurements — colour change, gas syringe, mass, water + gas
a) colour change: 1/time
b) gas syringe: measuring volume per second — cm3/s
c) mass: measure mass lost every 10 seconds — g/s
d) water +gas: volume of gas per 5 secs — cm3/s
Rate of reaction data
a) start: more reactant particles per unit volume, and more frequent collisions, more successful reactions, increase rate of reaction
b) part way through: fewer reactants per unit volume, less collisions
c) end of reaction: at least one of the reactants has been completely used up, still colliding but not successful.
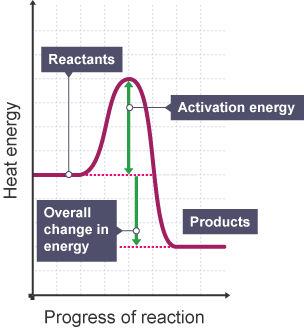
Exothermic Reactions
transfers energy to the surroundings, eg neutralisation, combustion. Bond making is exothermic. Products have less energy than the reactants.
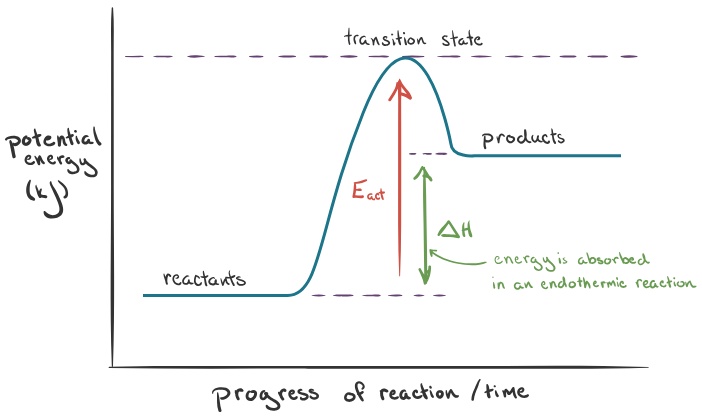
Endothermic
Takes in energy from the surroundings, eg photosynthesis. Bond breaking is endothermic. the products have more energy than the reactants.
catalysts
A chemical that speeds up a chemical reaction (by lowering the activation energy), but remains chemically unchanged. decreasing the activation energy means that more particles will have sufficient energy to overcome the energy barrier and react.