Aldehyde, Ketone,Carboxylic acid
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
53 Terms
similarity between Aldehyde, Ketone,Carboxylic acid
carbonyl compund
Other carbonyl groups than Aldehyde, Ketone,Carboxylic acid
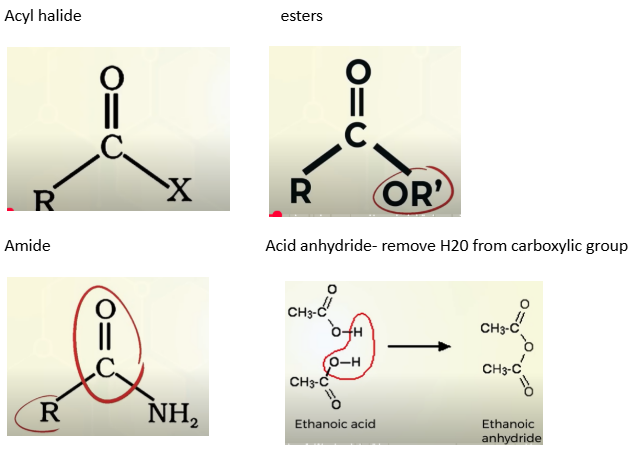
Acyl halide
esters
amide
acid anhydride
what is acyl group
R—C=O
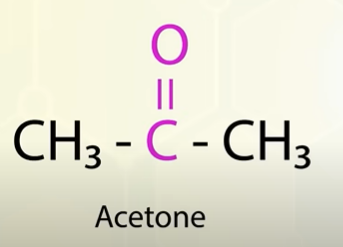
acetone structure
structure of acetophenone
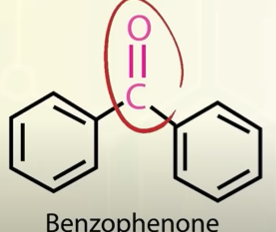
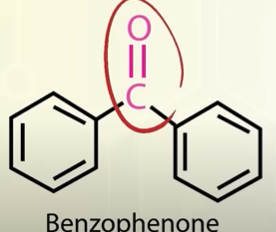
structure of benzophenone
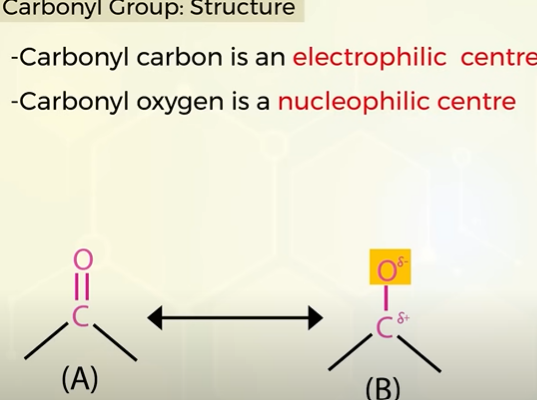
which is the elctrophillc and nucleophillic centres in the carbonyl group
methods to prepare aldehydes and ketones
oxidation of alcohol
dehydrogenation of alcohol
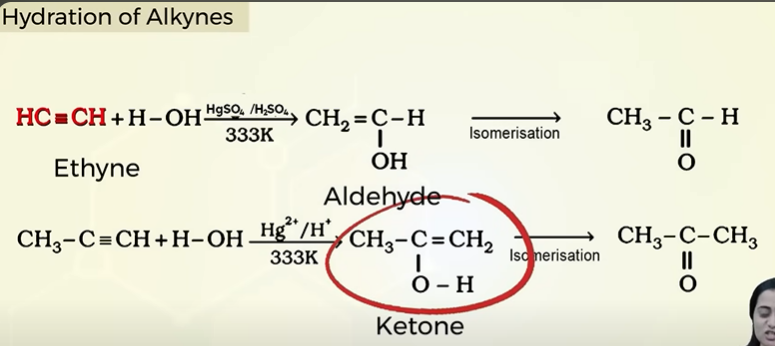
hydration of alkyne
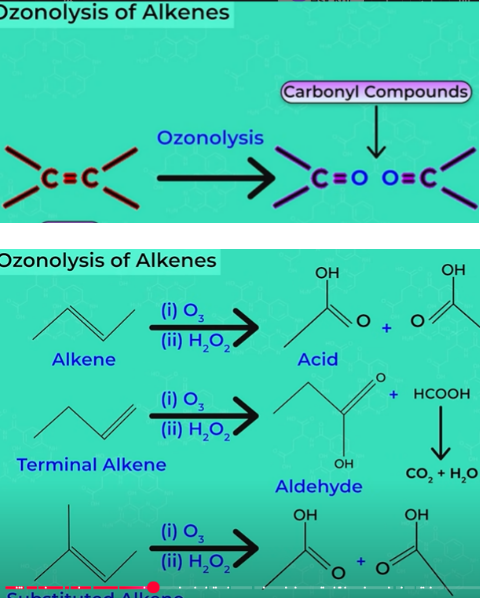
ozonolysis of alkenes
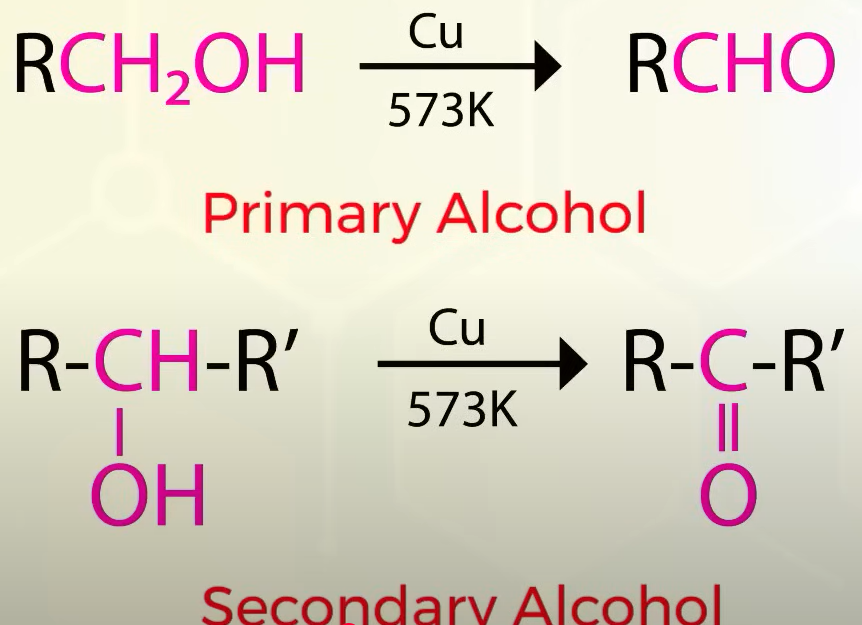
Oxidation of alcohol to obtain aldehydes and ketones
aka dehydrogentaion
H from O-H and from C-H is removed
alcohols on oxidation give aldehydes
aldehydes on oxidation give carboxylic acids
if a strong oxidising agent such as KmNO4 is used then we get carboxylic acid directly from alcohols
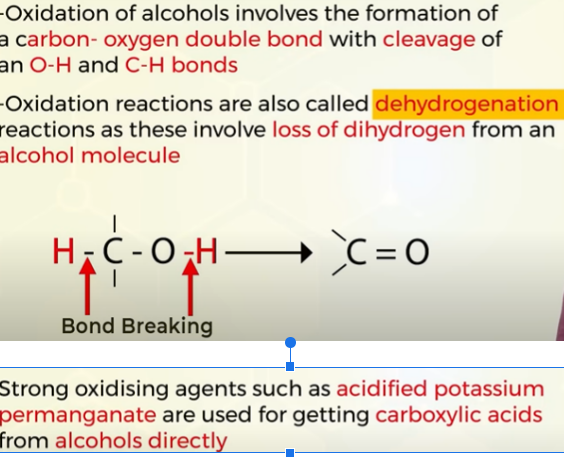
obtaining aldehydes and ketones from alcohols through oxidation
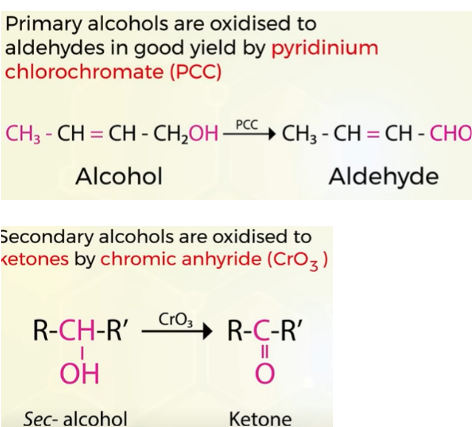
Mild oxidising agents
PCC(pyridinium chlorochromate) for primary alcohol to aldehydes
CrO3 (chromium anhydride) for sec alcohols to ketones
Why dont tertiary alcohols undergo oxidation?
We know that, Oxidation of alcohols takes place by removal of H form O-H and the alpha C
But in tertiary alcohols there is no H in the alpha carbon therefore they dont undergo oxidation
Dehydrogenation of alcohols
passing volatile vapours of alcohols through metals at a high temperature
dehydrogenation of:
primary alcohol- aldehyde
sec- ketone
tertiary they get dehydrated and give alkene
ozonolysis of alkenes
Hydration of alkynes
(alkynes are immisible in water)
only ethyne can make aldehyde
othere akynes make ketone
only in the presence of 333K and H2SO4/HgSO4
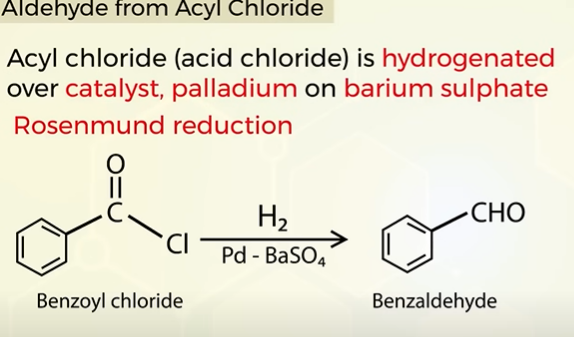
How to prepare only aldehydes

Aldehyde from acyl chloride (ROSENMUND REACTION)
Hydrogenated in the presence of a catalyst
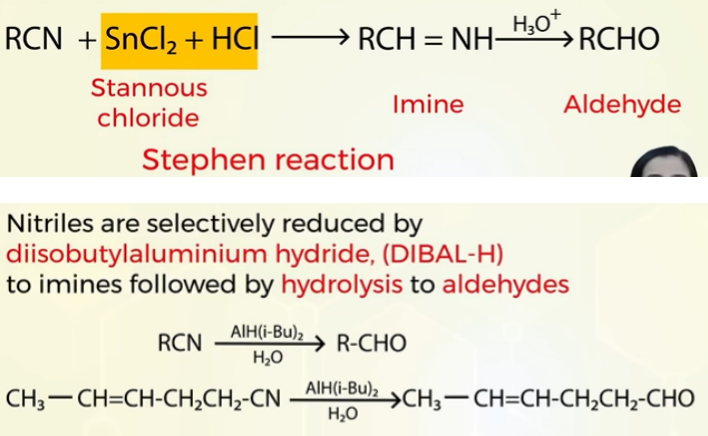
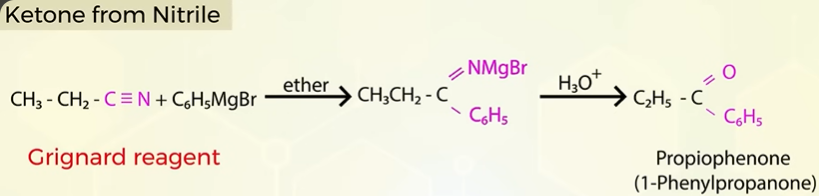
Aldehydes from nitrile (RCN) STEPHEN REACTION
instead of SnCl2 we can also use DIBAL-H( diisobyutylaluminium) for reduction
Difference between nitrile and cyanide
if CN is there in organc then called nitrile
else cyanide
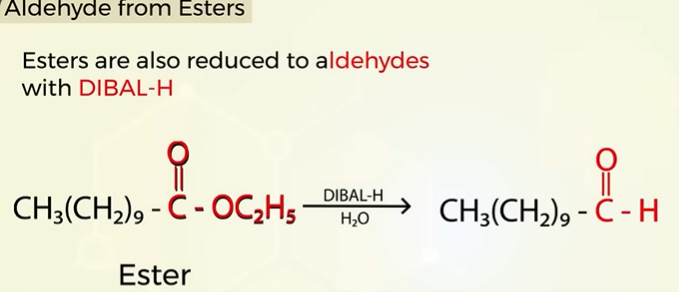
Aldehyde from esters
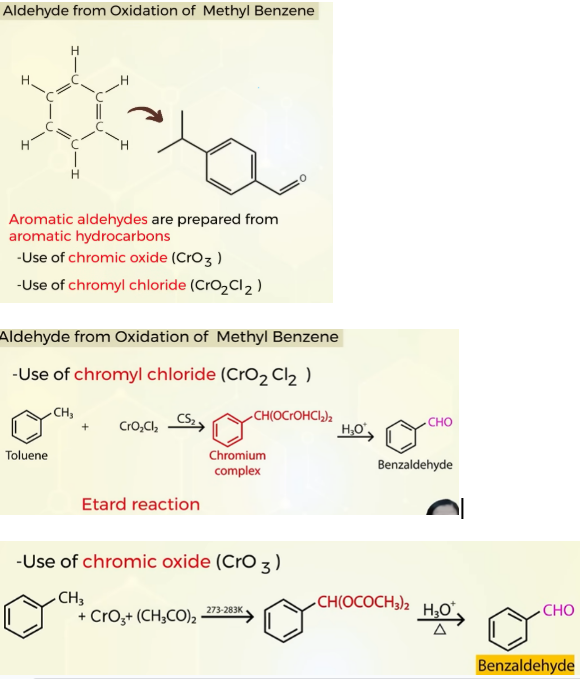
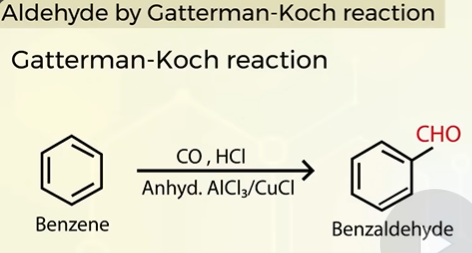
How to prepare aromatic aldehydes
Aromatic aldehydes are prepared from aromatic hydrocarbons through OXIDATION
you get benzaldehyde from toulene in the presence of chromyl chloride
ETARD REACTION :
you get benzaldehyde from toulene in the presence of chromyl chloride
use of CHROMIC OXIDE(CrO3)
In the presence of acetic anhydride
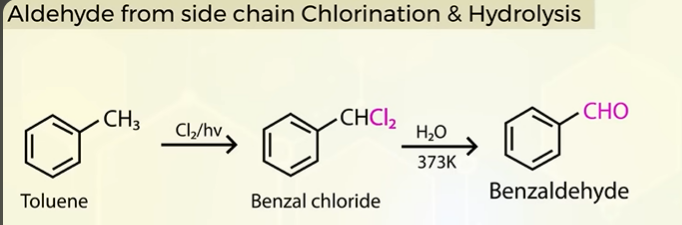
another method to get aromatic aldehydes from aromatic hydrocarbons
SIDE CHAIN CLORINATION AND HYDROLYSIS
THIS IS A COMMERCIAL WAY
preparation of benzaldehyde
GATTERMAN-KOCH REACTION
Preparation of ketones

Ketone from acyl chloride
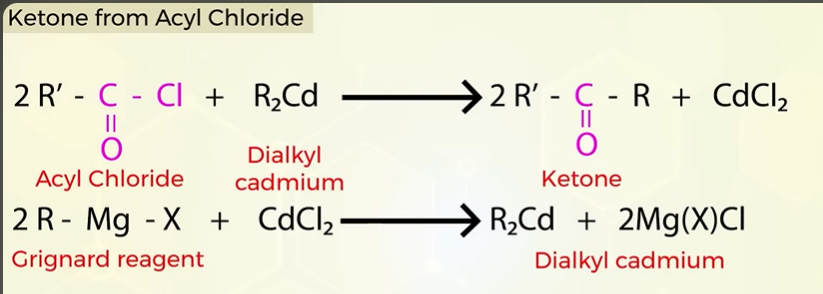
Ketone from Nitrile
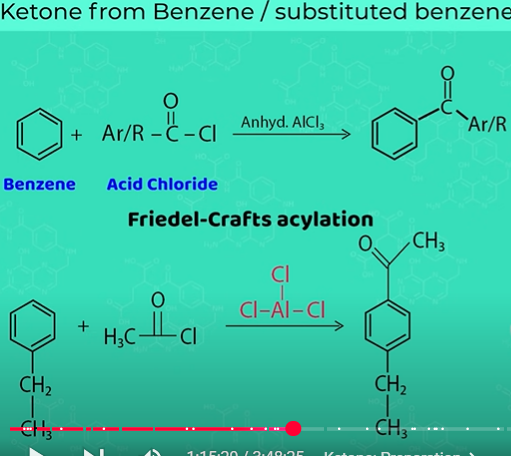
Ketone from benzene
Friedel crafts acylation
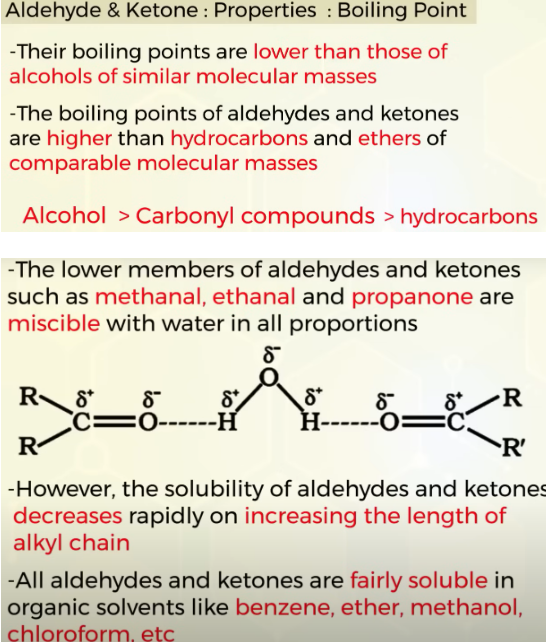
Properties of aldehyde and ketones
chck cw and book
are aldehydes or ketones more reactive in nucleophillic addition reaction?
aldehydes are more reactive in nucleophillic addition for the following reasons :
steric hinderamce in ketones
+I effect (give electrons) of alkyl groups on alpha carbon which reduce its partial postive, hence does not get affected by a nucleophile quickly
nucleophillic addition reaction of aldehydes and ketones
rxn with hydrogen cyanide
rxn with Hydrogen sulphite(NaHSO3)
with griganard reagent
with alcohols
with ammonia and derivatives
nucleophillic addition reaction of aldehydes and ketones with HCN( HYDROGEN CYANIDE)
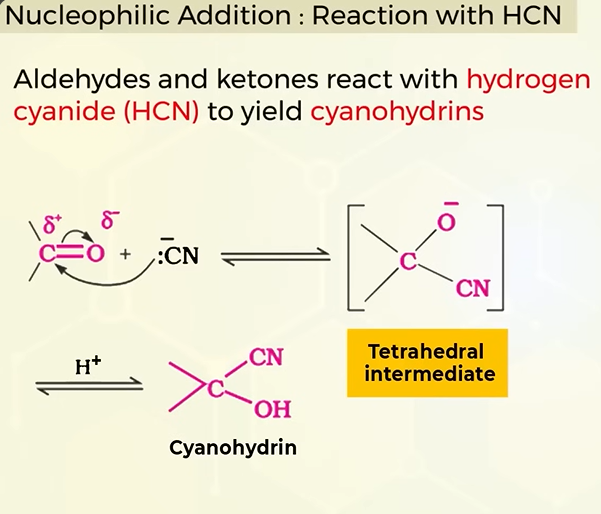
reaction of nucleophillic addition with pure HCN is slow. how do you fasten it?
by adding a base
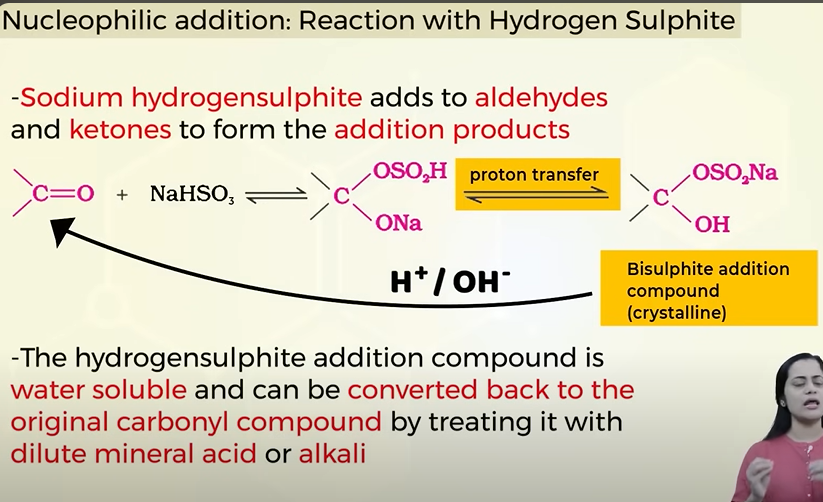
Nucleophillic addition of aldehydes and ketones with Hydrogen sulphite(NaHSO3).
Is it a reversible reaction? if yes then how to reverse
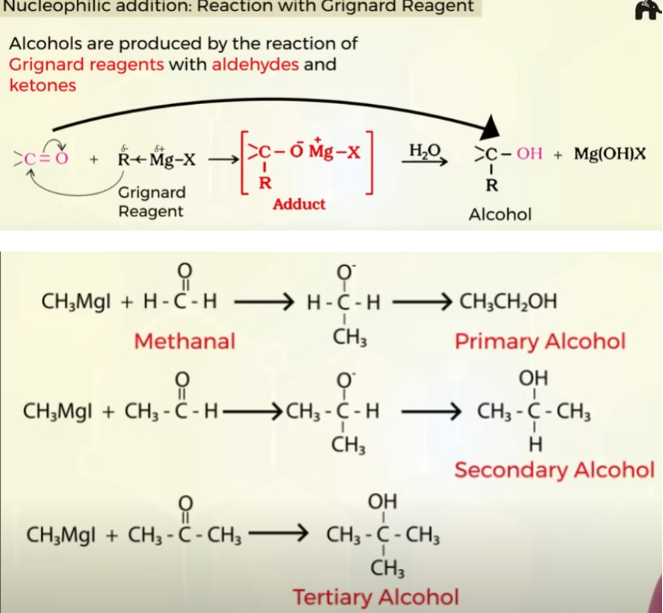
Nucleophillic addition of aldehydes and ketone with R-Mg-X (Griganard reagent).
alcohol formed
Nucleophillic addition of aldehydes and ketone with alcohols and whats the special case
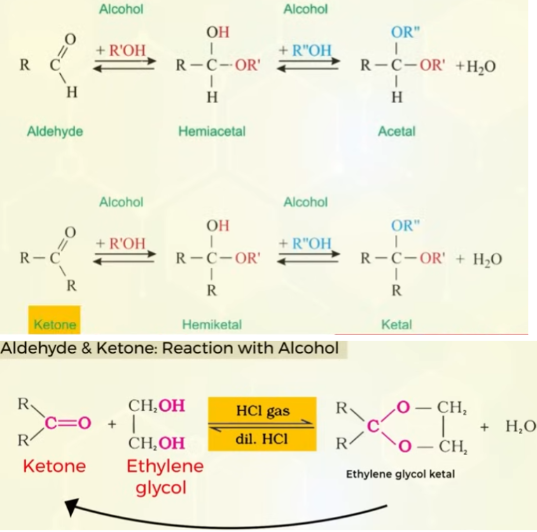
Nucleophillic addition of aldehydes and ketone with alcohols with ammonia and derivatives
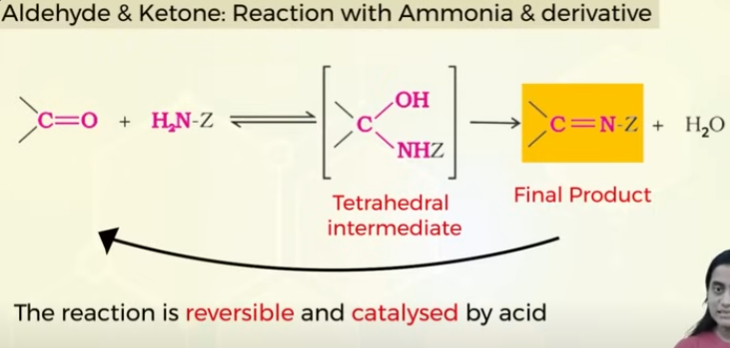
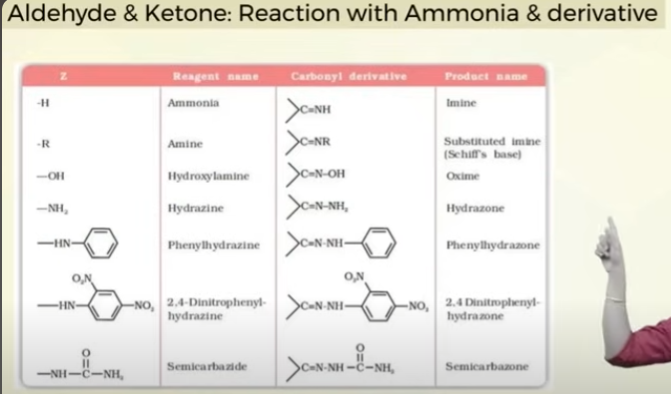
learn reaction of aldehydes and ketones with ammonia and its derivatives whith diff substituents of Z
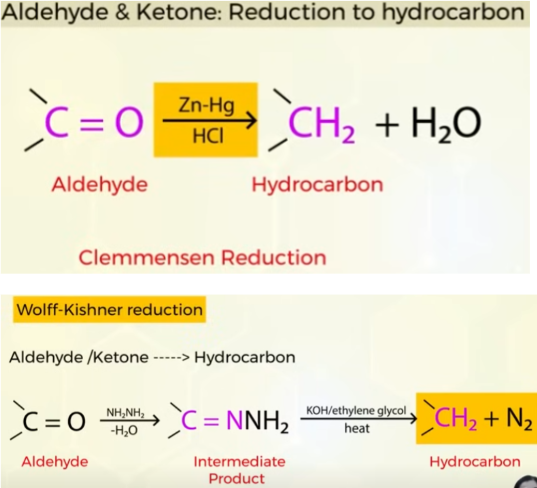
REDUCTIONS OF ALDEHYDE AND KETONES
To alcohols
to hydrocarbons (wolf and clemmensen)
reduction of aldehydes and ketones to alcohol
reduced using reducing agents like (LiAlH4) AND NaBH4
Or catalytic hydrogenation
reduction of aldehydes and ketones to hydrocarbons
Clemmensen reaction
Wolff-Kisher reduction
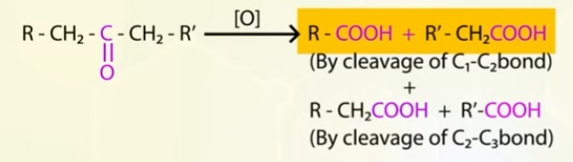
Oxidation of aldehydes and ketones to carboxylic acids
Oxidation of aldehydes and ketones takes place differently.

Oxidation of ketones
you get mixture of carboxylic acids
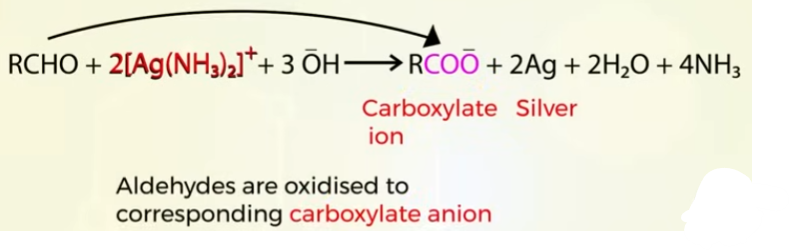
Oxidation of aldehydes and ketones by Tollens test
can be used to find out if the carbonyl compound is aldehyde or a ketone
if the reaction takes place it is aldehyde
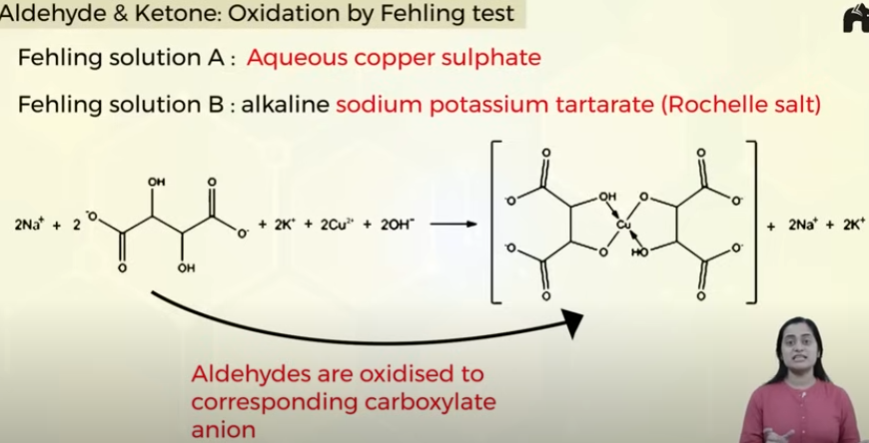
Oxidation of aldehydes and ketones by Fehling test
reaction takes place for aldehydes but not ketones
aromatic aldehydes do not respond to fehlings test