NMR Studies of Intermediates
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
50 Terms
What strategies can you use to study a reaction mechanism?
Synthetic studies: varying ligands/metals to control reactivity. Can stabilise and detect reaction intermediates.
Kinetic studies: varying concentration and temperature to provide kinetic stability, stabilising intermediates.
Isotope studies:2D and 13C labels improve NMR sensitivity and can add diagnostic couplings.
What is DFT used for?
Density Function Theory- sed to calculate stability of intermediates and predict outcomes.
Good for neutral systems as charged complexes with lots of solvation are hard to examine.
What is a potential energy surface?
It reflects the change in energy as a function of atomic coordinates related to the reaction.
Potential energy is plotted as a function of the reaction coordinate.
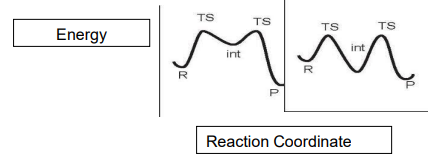
How do you interpret potential energy graphs?
The energy barrier is the gap between the TS and previous state (black lines)
The ΔG is the gap between the energies of the reactant and product.
The profile on the left shows a less stable intermediate as it has a higher energy, therefore it has a lower lifetime.
What is the M-H bond distance?
Around 1.5-1.7 A
Why are hydrides difficult to see using X-ray diffraction?
There is a lack of electron density on the hydride vs the adjacent atoms (especially the metal).
Neutron diffraction is more accurate.
What is the difference between hydrogen containing complexes of late and early TMs?
Late TMs tend to be more ‘protic’
Early TMs tend to be more ‘hydridic’
This is important when considering chemical shifts, as hydrides will appear more negative.
How strong is a M-H bond?
They are very strong, around 240-400 kJ/mol.
A M-C BDE is around 100-120, therefore an M-H is relatively much stronger.
How do M-H bonds appear in IR?
They are IR weak, therefore hard to spot. The IR shift depends on the location of the hydrogen.
An M-H is around 2100-1600 cm-1.
A M2(μ-H) is around 1600-800 cm-1 and is broader.
What is a hydride?
A H- anion.
Is a 2- donor
Can be bridging or terminal.
Where does a M-H bond appear in NMR?
Around 12 to -50.
How does the trans ligand affect the chemical shift of the hydride?
If there is a soft ligand in the trans position, the chemical shift is less negative. If there is a hard ligand, the chemical shift is more negative.
CO is a soft ligand.
Cl is a hard ligand.

How do d-electrons affect the chemical shift of the hydride?
A complex with a high d electron count means the metal is more electron rich, making the hydride more basic/nucleophilic.
The hydride will have a more negative chemical shift.
A low d count means the metal is electron poor, so the hydride acts more acidic.
The hydride will have a less negative shift.
How do you form metal hydride complexes?
Reacting a metal carbonyl and H2 will form a hydride complex.
These complexes can be quite acidic, so can dissociate.
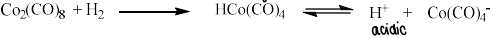
What are the bonding interactions between a metal and a dihydrogen ligand?
The dihydrogen ligand acts as a sigma donor, donating 2 electrons to the metal.
The dihydrogens acts as a sigma* acceptor, the filled d orbitals of the metal donate into the empty sigma* orbitals of the dihydrogen.
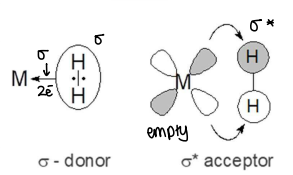
How does a dihydrogen ligand turn into a dihydride ligand?
If the metal is electron rich, it can give lots of electron density to the H-H sigma* bond.
This interaction increases the M-H bond strength, but decreases the H-H bond strength.
If the electron density on the H-H bond becomes too high, the H-H bond will break.
How do you tell the difference between a dihydrogen and dihydride isomer in an NMR?
For a dihydrogen:
1H chemical shift is -4.20 ppm
31P chemical shift is 33.5 ppm
For a dihydride:
1H chemical shift is -2.15, -4.50 ppm
31P chemical shift is 30.8, 39.5 ppm
A dihydride has 2 chemical shifts, whereas a dihydrogen only has one.
How do you tell the difference between a dihydrogen and dihydride using bond lengths?
The H-H bond length for a dihydrogen is short, whereas a dihydride is much longer. As the backbonding from the metal centre increases, the bond length increases.

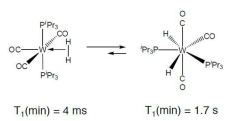
How does measuring T1 differentiate dihydrogens and dihydrides?
T1 is the dipolar relaxation of the hydrogen nucleus. A short T1 means the nucleus relaxes quickly, whereas a long T1 means it relaxes slowly.
If the two hydrogen nuclei are closer together, they have stronger dipolar coupling and so relaxation is faster (short T1).
A dihydrogen complex has a shorter T1 than a dihydride.
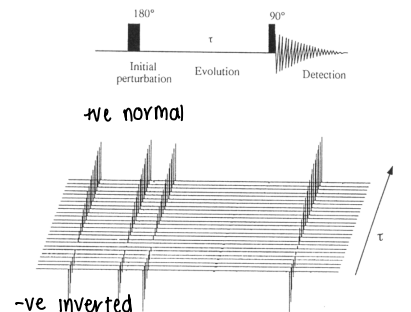
What is the T1 experiment?
A pulse is applied to the nuclei, inverting them by 180o so they point against the magnetic field.
The magnetization begins to recover over time (𝜏).
A 90o pulse is applied, measuring how much the magnetization has recovered.
This is used to calculate T1.
How is 1JHD coupling used to differentiate between a dihydrogen and dihydride?
Free HD has a coupling constant of 43.2 Hz (quite large).
A regular H-H dihydrogen complex appears as a single peak as the H atoms are equivalent.
A H-D dihydrogen appears as a triplet, it has a coupling constant of around 28 Hz.
Dihydrides show no (or very small) H-D coupling, it is usually 1 Hz or less and is hard to measure.

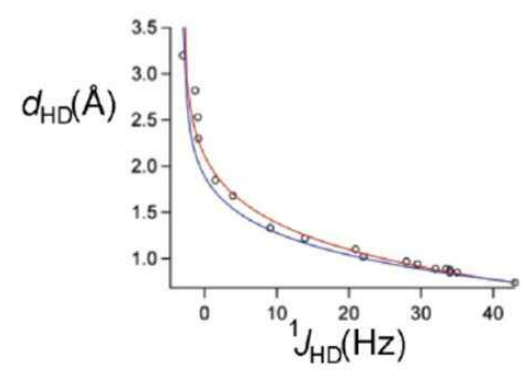
What is the correlation between 1JHD coupling and bond distance?
There is an empirical correlation, the coupling constant can be used to predict the bond length.
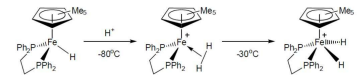
How can you transform a neutral hydride into a dihydrogen complex?
Protonate using an acid with a weak conjugate base.
How does R3Si-H bond to a metal centre?
Similar to a dihydrogen.
The Si-H bond is a 2 electron donor.
How does the bond distance and JSi-H constant differ in free silane and silane in a metal complex?
The coupling constant in free silane is much larger (by a factor of 10x) as the Si-H bond is much stronger. The Si-H bond in a metal complex is weakened by the metal backbonding.
The Si-H bond distance in free silane is smaller due to the stronger bond.
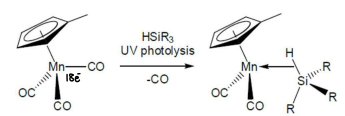
How are metal silane complexes formed?
Photochemistry is a good way to form a 16 electron complex. HSiEt3 is then added, forming the 18 electron complex.
It is expected that the weak Si-H bond will be broken.
How is light used to form a 16 electron complex?
When light is applied to a stable complex, electrons are moved into anti bonding orbitals. This leads to ligand loss via reductive elimination. The desired ligand can then be added.
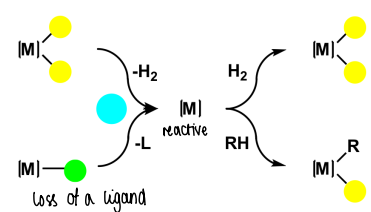
What is dynamic behaviour?
When positions of ligands swap places, causing coalescence in the NMR spectrum.
It is a function of temperature, cooling down the sample will slow down dynamic behaviour, making NMR easier to interpret.

How does a silane metal complex appear in NMR?
The H-Si coupling in a η2 silane is heard to measure.
The coupling between the metal (Rh) and the hydride depends on the O.S. of the metal.
In a H decoupled spectrum, the silicon will couple to the metal (if it is NMR active).
How do metal complexes with a cationic alkyl ligand appear in NMR?
The J13C-H coupling in a CH3 would normally be around 130 Hz, whereas in this complex it is around 61 Hz due to stabilisation.
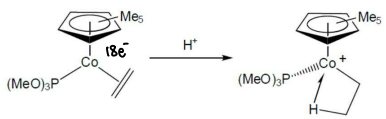
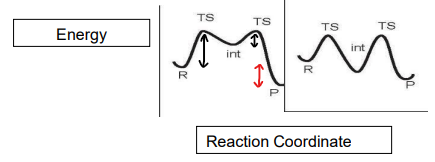
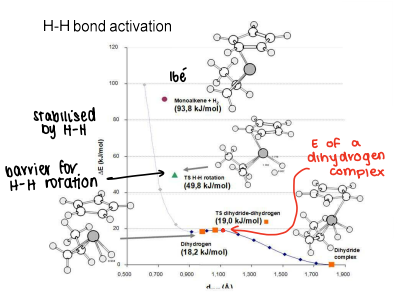
How does energy change as a function of H position when H2 is added to a monoalkene complex?
The monoalkene is high energy as it is a 16 electron complex.
When H2 is added, the complex is stabilised, however there is a barrier for rotation.
The energy slightly increases as the TS for dihydride conversion forms.
The energy drops as the dihydride complex forms as it is more stable.
The energies are determined by DFT.
What do metal complexes look like in UV spectroscopy and why?
The UV spectrum becomes more blue shifted as there is an increase in O.S. of the metal.
The electrons move to lower energy with higher O.S., therefore more energy is needed to promote them. This makes it blue-shifted.
Low O.S. are red-shifted.
How is NMR used with photochemistry?
NMR probe used, NMR via in-situ photolysis inside the probe.
Photolysis can cause different products to be formed, forming multiple NMR signals.
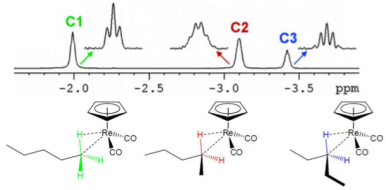
Do complexes prefer to coordinate to CH2 or CH3?
CH2, this is because it can form a stable 18 electron intermediate.
Coordinating to a CH2 gives a more stable bonding rearrangement.
A CH3 has no available empty orbital, so would likely rearrange itself.
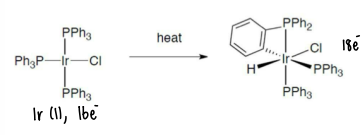
How is the C-H bond activated to allow the metal to insert itself?
Heating can be applied to allow a 16 electron metal to insert into the C-H bond, forming a new M-H and M-C bond. The new 18 electron complex will be more stable.
What determines the intensity of a resonance in NMR?
Related to the difference in population of the two states:
The H nucleus interacts with a magnetic field, forming two different nuclear spin orientations separated by a specific frequency.
The energy difference is small and so is the population difference.
A small population difference means a weak signal.
In NMR, what does the hydride chemical shift depend on?
Depends on the trans ligand.
Hard ligand- very negative.
Soft ligand- less negative.
Another NMR active nuclei can split the signal.
How do you interpret a 2D 1H-13C spectrum?
It looks at chemical exchange and through space interactions.
Cross peaks indicate exchange when positive, and through space interactions when negative.
What is DNP?
Dynamic nuclear polarisation. An electron in a stable radical is used to polarise nuclei at low temperatures under microwave irradiation. When the sample thaws there is a strong NMR signal.
This is due to a change in population difference that exceeds the normal value (it is hyperpolarised).
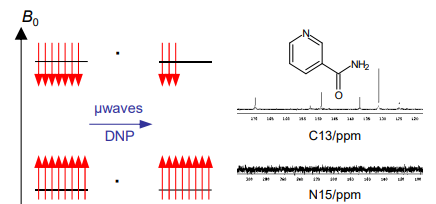
How does DNP work?
The sample is frozen with a radical at 1.3K
Microwave irradiation occurs at 3.35 T, allowing cross polarisation and causing a change in population difference.
Rapid thaw and injection follow, the NMR is recorded.
The polarisation can also be transferred to a nucleus.
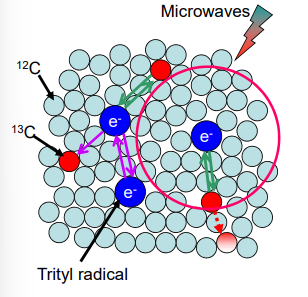
What radicals can be used for DNP?
Need a stable radical that is soluble in water.
Can use steric stabilisers.
How can DNP be used to follow an inorganic reaction?
Can hyperpolarise an alkyne, this means it will insert into a metal hydride, forming a vinyl.
The site that is 13C labelled will cause splitting and have a strong signal due to the DNP.

What are the quantum mechanical properties of hydrogen?
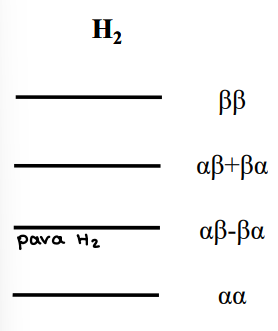
A H2 atom has two hydrogen nuclei, which are protons. They have I = 1/2, and ml can equal ½ (a) or -1/2 (B).
The spin combinations of the two atoms can be represented by a or B, e.g. aB or aa.
H2 is a fermion, therefore the wavenumber must be antisymmetric under particle exchange.
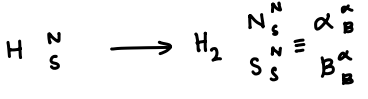
When is the wavenumber for H2 antisymmetric?
Translational, vibrational and electronic contributions are always symmetric, so the product of the rotational and nuclear spin wavefunctions must be antisymmetric.
When the particle spins are swapped, if the original spin combination must be multiplied by -1 to achieve the new combination, it is antisymmetric
If multiplying by 1, it is symmetric.

What is the parahydrogen effect?
The para-isomer of H2 has an antisymmetric spin configuration (aB-Ba).
This must be paired with symmetric rotational states (J= 0,2,4).
This means the para hydrogen form is of lower energy as ortho H2 has the lowest J value of 1.
Which H2 isomer is favoured and why?
The population difference is temperature dependent- at low temperature more para isomer is present as it is more stable.
Interconversion is spin forbidden (needs to change 2 quantum numbers).
When do para and ortho isomers of H2 convert?
Interaction with a paramagnetic material enables rapid interconversion of spin states.
On removal of the catalyst, H2 is slow to reestablish spin state thermodynamic equilibrium as interconversion is spin forbidden.
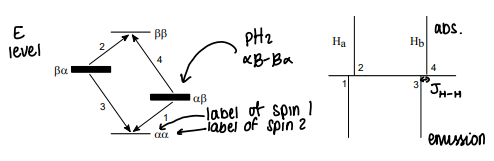
What do the energy levels for H2 look like?
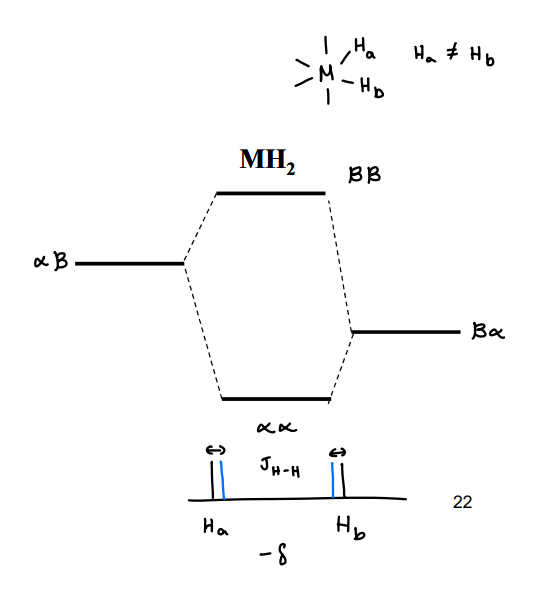
What do the energy levels for para MH2 look like?
When the para H2 reacts with a metal, it forms two seperate hydrogens that are no longer identical. This makes distinguishable.
There will be two separate peaks in NMR, with a small spin-spin coupling constant.
What is PHIP NMR?
Para-Hydrogen Induced Polarisation, para hydrogen can be used to enhance an NMR signal.
The spin state of the hydrogen atoms is preserved, giving a nonequilibrium spin population in the product.
There are two pairs of signals, the emission signals point up and the absorption signals point down.
Must break the symmetry of the hydrogens or the enhanced signals will cancel out.