microbiology exam 4
1/241
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
242 Terms
disease
a condition where normal structure and/or function are damaged or impaired
infection
invasion of pathogn or parasite that leads to disease
signs (evidence for infection)
things that can be directly measured by a clinician (e.g. blood count)
symptoms (evidence for infection)
things felt by patient that cannot be clinically measured (e.g. nausea)
syndrome
groups of signs and symptoms that help indicate a particular disease
some symptoms may be asymptomatic = meaning that only signs can be observed through correct testing
e.g. patient w/ herpes and no symptoms
infectious (classification for diease)
disease is directly affected by pathogen(s)
communicable (classification for disease)
capable of spreading person-to-person
latrogenic (classifications of disease)
acquired as result of a medical procedure
nosocomial (classification for disease)
acquired from a hospital setting
zoonotic (classifications of disease)
acquired from animal, usually vertebrate
non-communicable (classification of disease)
obtained from non-living thing such as soil of contaminated object
non-infectious (classification of disease)
not caused by pathogen
WHO’s international classification of disease (ICD)
used globally to classify and monitor diseases
healthcare professionals (doctors, nurses, researchers, epidemiologists, etc.)
ICD used by
periods/stages of disease
incubation
prodromal
illness
decline
convalescence
incubation (stage one of disease)
initial entry of pathogen; replication begins
prodromal (stage 2 of disease)
replication continues; host starts to show signs and symptoms
illness (stage 3 of disease)
signs and symptoms are most severe in host
decline (stage 4 of disease)
pathogen now starts to decrease; host’s immune system is weak and vulnerable to secondary infection
convalescence (stage 5 of disease)
host starts to recover
patient is contagious is which stages of disease?
all stages
koch’s postulates (developed in 1884)
set of standards that must be met
The suspected pathogen must be found in every case of disease and not be found in healthy individuals
The suspected pathogen can be isolated and grown in pure culture
A healthy test subject infected with the suspected pathogen must develop the same signs and symptoms of disease seen in postulate one
The pathogen must be re-isolated from the new host and must be identical to the pathogen in postulate 2
koch’s wrong assumptions
pathogen are only found in diseased individuals
all subjects are equally susceptible to infection
all pathogens can be grown in culture
molecular koch’s postulates
postulates improved with molecular methodologies
overcame some of koch’s limitations
identifies gene instead of pathogen
pathogenicity
ability of pathogen to cause disease
virulence
degree of pathogenicity
continuum among many pathogen types
ex. highly virulent - Bacillus anthracis induces severe signs and symptoms
ex. low nutrient - rhinovirus induces low signs and symptoms
pathogens - primary and opportunistic
some are automatic pathogens, some are not
primary pathogen: enterohemorrhagic E. coli (mainly due to Shiga toxin)
opportunistic pathogen: candida albicans; UTI caused by E. coli
Drugs, resident microbiota, genetics, and age can all influence susceptibility to disease
acute v. chronic disease
illness period can be variable
acute disease: relatively short (hours, days, week)
chronic disease - longer time (months, years, lifetime)
latent disease - comes in episodes; pathogen replicates when disease is active
virulence curve
virulence can be modelled in controlled experiments
median infectious dose - no. of pathogens required to infect 50% of population
median lethal dose - no. of pathogens required to kill 50% of the population
medican infectious dose (virulence curve)
how mant pathogens will infect 50% of the population?
median lethal dose (cirulence curve)
how many pathogens to kill 50% of the population?
virulence curve - brain training on reading graphs
The higher the value (more pathogens), the WORSE the infection is. (200 pathogens to infect/kill 50% of the population is worse than 400 pathogens to infect 50% of the population)
stages of pathogenicity
exposure to host
adhesion
invasion and colonization
infection
exposure to host (stage of pathonenicity)
can occur in many ways; pathogens must be exposed to portals of entry (eyes, nose, throat, mouth, vagina, anus, urethra, broken skin, needle, palcenta, insect bite, etc.) to begin adhesion (also known as trophism)
some portals are worse than others (e.g. mucosa)
TORCH infections (infect pregnant women)
pathogens that can cross planential barrier as portal of entry
Tocoplasmosis
O (syphilis, chickenpox, hepatitis B, HIV, fifth disease - erythema infectiosum)
Rubella (german measles)
Cytomegalovirus
Herpes
adhesion (step of pathogenicity)
pathogens have varying capability of colonization.
Adhesion factors:
molecules/structures that bind to certain host receptors
biofilm- production of community glycocalyx
invasion (step of pathogenicity)
occurs when colonization is established.
pathogens generally produce toxins to allow further colonization into body/tissue
virulence plays role in degree of invasion
Ex. Helicobacter pylori urease production
obligate intracellular invade via
endocytosis and evasion of host immune defenses
invasion mechanisms
effector proteins are secreted to trigger entry (e.g. salmonella and shigella spp.)
surface proteins allow for binding to host cell (trojan horse approach)
some pathogens are able to survive
lysosome that engulf (ex. Mycobacterium tuberculosis and listeria)
some pathogens can evade
phagocytosis of WBC
listeria - can lyse phagosome before fusing with lysosome
mycobacteria tuberculosis - prevent fusion of phagosome with lysosome
infection (stage of pathogenicity)
multiplication leads to established host infection.
types of infection
local infection
focal infectionp
systemic infection
local infection
small area on the body
focal infection
pathogen or toxin spreads to secondary location
systemic
occurs throughout body (ex. septicemia)
primary infections can lead to
secondary infection of different pathogen
Ex. HIV lowers immune system and opens door for yeast and others; rhinoviruses can lead to bacterial pneumonia
virulence factors dictate how
severe and extensive a disease is.
some have more than one = more virulent
examples
adhesion factors (adhesion)
exoenzymes (invasion)
toxins (invasion)
immune evasion (invasion)
adhesins - virulence factors
Proteins that aid in attachment to host cell receptors
commonly found in fimbriae or pili
can initiate biofilm formation in some species
Ex. Stereptococcus pyogenes produces Protein F to adhere to respiratory epithelia on the back of the throat (pharynx)
exoenzymes (virulence factors)
extracellular enzymes used to invade host tissues
examples: glycohydrolases, nucleases, phospholipases, proteases
toxins (virulence factors)
poisons that cause host cell toxigenicity
endotoxins - lipolysaccharides (only gram - ) that triggers host inflammatory responses; can cause severe fever and shock
exotoxins - proteins mostly produces by Gram (+); targets receptors on specific cells
intracellular targeting (exotoxin)
with A and B regions for activity and binding; Ex. diptheria and botulinum toxin
Membrane disrupting toxin (exotoxin)
aka phospholipases that degrade bilayer membrane; Ex. Bacillus anthracis and rickettsia spp.
S. pyogenes produces streptolysin, enhancing invasion to cells
superantigen toxin (exotoxin)
trigger excessive producation of cytokines by immune cells; Ex. staphylococcus auerus and toxic shock syndrome
host evasion (virulence factors)
mechanisms to evade phagocytosis
examples:
capsules that enlarge bacterial cell so phagocytes cannot engulf pathogens
proteases digest host antibody molecules
mycolic acid in acid fast bacteria (M. tuberculosis) helps evade phagolysosomes
coagulase pos. microbes can coagulate blood cells to keep immune cells out of reach
alteration of cell surface proteins to hide from immune cell recognition
virulence in viruses
some properties are similar to bacteria (adhesions and antigenic variation)
example:
HIV glycoprotein 20 for binding to CD4 T-cells
influenza virus’ high mutation of envelope spikes allows for antigenic variation
virulence in fungi
many properties are also similar to bacteria (adhesions, proteases, and toxins)
example
capsule (+) cryptococcus spp. can cause pneumoniae and meningitis
mycotoxins produced by Claviceps purpurea and Aspergillus spp. that contaminate grains and other staple crops
virulence in protozoans
unique features for attachment-
Giadfia lamblia uses adhesive disk of microtubules to attach to intestines
plasmodium falciparum quickly changes adhesive protein fr RBC’s to avoid immune recognition; causes chronicity in malaria patients
virulence in helminths
tissue penetration commonly achieves w/ proteases (e.g. worms that burrow into skin)
roundworms produce cuticle to last longer against host defense assaults
schistosoma mansoni degrades host antibodies to halt immune defense
focal infection
a localized infection that spreads to other parts of the body, potentially cuasing systemic disease.
local infection
an infection confines to a specific part of the body, like a urinary tract infection or a skin infection, and doesn’t spread throughout the body.
secondary infection
an infection that occurs during or after treatment for another primary infection
systemic infection
an infection that has spread throughout the body often through the bloodstream
causes of superinfections
the growth of resistant organisms (bacteria, lichens, or fungi) that are normally held in check by the forms of bacteria normally present in the oral and intestinal tracts of the host animals.
TORCH pathogens
infectious agents that can be transmitted from a mother to her fetus during pregnancy.
(Toxoplasmosis, Others (syphilis, Zika virus, malaria, HIV), Rubella, Cytomegalovirus, and Herpes sumplex virus)
ID50 vs. LD50
ID50 is the infectious dose that will cause 50% of people exposed to a pathogen to become contaminated.
LD50 is the lethal dose that will kill 50% of people.
Limulus amoebocyte lysate (LAL) test and endotoxins
LAL test is widely used in vitro assay for detecting bacterial endotoxins. It relies on the coagulation of amebocyte lysate from horseshoe crabs in the presence of endotoxins, a process that can be measured quantitatively or qualitatively
facultative intracellular pathogens
microorganisms, primarily bacteria, that can replicate both inside and outside host cells.
obligate intracellular pathogens
microorganisms that can only survive and reproduce inside the cells of a host.
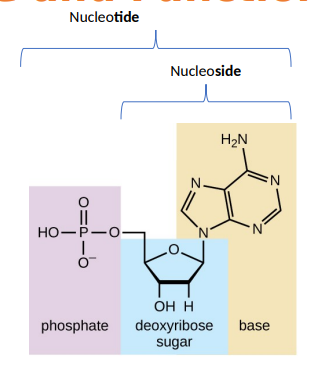
what makes up a DNA nucleotide
a phosphate group, a five-carbon sugar called deoxyribose, and a nitrogenous base. the four nitrogenous bases in DNA are adenine (A), guanine (G), cytosine (C), and thymine (T).
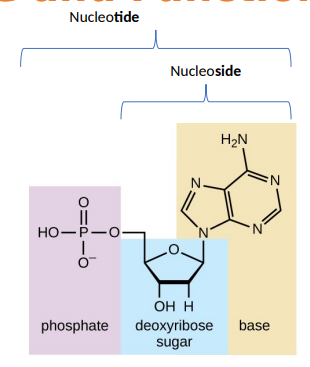
what makes up a DNA nucleoside
a five-carbon sugar called deoxyribose and a nitrogenous base (A, G, C, T).
NO PHOSPHATE GROUP
parts of DNA
deoxyribose sugar backbone
nitrogenous bases
2 functions of DNA
info for cell functions
info for cell replication
main player in cellular (central dogma)
purines
adenine and guanine
double-ring structures
pyrimidines
cytosine, thymine, and uracil
have a single-ring structure
binding location of DNA binding proteins
major groove of DNA because it exposes more functional groups for recognitions of specific base pairs.
they can also bind to the minor groove or interact with other DNA features like origins of replications, centromeres, and telomeres.
phenotype
observable characteristics resulting from genotype interaction
expression of a set of genes
genotype
an organism’s genetic makeup.
collection of all genes in a cell
steps of PCR
denaturation
DNA is heated to 95 celsius to separate it into a single strand, breaking the hydrogen bonds between base pairs.
annealing
the temperature is loweres to 55-72 celsius to allow short DNA sequences calles primers to bind to a single-stranded DNA at specific target locations
extension
the temperature is raised again to 72 celsius allowing the DNA polymerase enzyme to extend the primers by adding nucleotides and synthesizing new DNA strands, complementary to the template
vertical gene transfer
the passing of genetic information from a parent organism to its offspring during sexual or asexual reproduction
horizontal gene transfer
the movement of genetic material between organsims, not through reproduction from parent to off-spring.
DNA v. RNA
DNA is double-stranded, with a stable long-term storage role
RNA is typically single=stranded and more involved in the process of protein synthesis
RNA synthesis and ribosome assembly location
nucleolus, a specialized region within the nucleus.
super coiling
the winding and unwinding of the DNA double-helix beyond its typical helical structure, creating a higher-order structure
peptidyl transferase
a ribozyme, meaning it’s an RNA molecule with enzymatic activity, located in the ribosome.
plays a crucial role in protein synthesis by catalyzing formation og peptide bonds between amino acids.
facilitates the transfer of a growing polypeptide chain from a tRNA molecule in the P site to the amino acid attached to a tRNA in the A site.
differences between RNA types
mRNA: linear, single-stranded that carries genetic information from DNA to the ribosome
rRNA: forms a major compoenent of ribosomes and plays a structural and catalytic role in protein synthesis
tRNA: smaller, L-shaped molecule that carries specific amino acids to the ribosome for protein synthesis
ribosomal RNA (rRNA)
make up ribosomes with proteins
messenger RNA (mRNA)
carries message from DNA to ribosome
transfer RNA (tRNA)
carries amino acid to growing peptide chain at ribosome
Joachim Hammerling- discovery of the nucleus (hereditary information)
Used single cell agla to show important of nucleus in propagation and survival.
showed that the nucleus in the foot and would kill algal cell if removed.
proposed nucleus was source of herediary information.
A. mediterranea foot was grafted with cap of other species A/ crenulata but did not take on new algal traits.
Beadle and Tatum (1941)- one gene-one enzyme hypothesis
used mold Neurospora crassa
mutant spores were induced with x-ray exposure
mutants were examines to determine which amino acid(s) they could/couldn’t produce
later was revised to one gene-one enzyme polypeptide (because not all code enzymes).
frederick griffith (1928)- hereditary info can be horizontal and vertical
showed heredditary info can be shared to cells of same generation by demonstrating bacterial transformation - bacteria pick up external DNA
his model worked with pathogenis (S) and non-pathogenic (R) strains of Streptococcus pneumoniae
DNA was picked up by (R) strain after (S) strain was heat killed
Avery, MacLeod, McCarty (1941)- DNA was transforming component
expanded on Griffith experiment by degrading specific enzymes
then attempted a transformation to see the component responsible (protein, RNA, or DNA)
transformation ONLY occurred when DNA was available after heal killing the (S) strain
Alfred Hershey and Martha Chase (1952)- DNA as genetic material
used bacteriophage with radioactive sulfur (proteins) or phosphorus (DNA) to infec E. coli.
phosphhorus labelled phages created new phages with the label in E. coli.
sulphur labelled phased remained outside E. coli. phage inside had no labelled sulphur
conjugation
use of pilus to transfer genes cell-to-cell
tranformation
naked DNA is taken up by cell
transduction
genes are transferred via virus

central dogma
describes the flow of genetic information from DNA to RNA to proteins.
DNA > transcription > RNA > translation > proteins
okazaki fragments
used for lagging strand (3’ to 5’) in DNA replication.
short, newly synthesized strands of DNA formed on the lagging strand during DNA replication.
they are created discontinuously and are later joined together by the enzyme DNA ligase to form a complete, continous DNA strand.
transcription in eukaryotes
RNA polymerasses
Addition of 5’ cap and poly A tail
removal of introns (mRNA splicing)