Chemistry topic 5: energy changes
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
exothermic
in an exothermic reaction, the energy released from forming new bonds in greater than the energy needed to break existing bonds therefore energy is released into the surroundings in the form of heat
endothermic
in an endothermic reaction, the energy released from forming new bonds is less than the energy needed to break existing bonds therefore energy is taken in from the surroundings in the form of heat
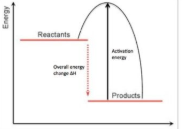
endothermic energy profile
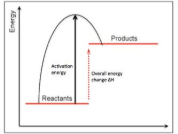
exothermic energy profile
cell
a device that generates electrical energy from chemical energy, it contains two electrodes immersed in an electrolyte
battery
a device that stores chemical energy and converts it into electricity, consists of two or more cells connected in series
non-rechargeable/non-renewable batteries
the chemical reactions stop when one of the reactants has been used up eg. alkaline battery
rechargeable/renewable batteries
-the chemical reactions are reversed when an external current/potential difference is supplied eg. hydrogen fuel cell
-can store less electricity the more charging cycles they go through and will eventually need replacement
-no dangerous fuels required, can produce a greater p.d than a hydrogen fuel cell
energy change =
sum of bonds broken - sum of bonds made
hydrogen fuel cell
-electrochemical cell
-contains hydrogen and oxygen that have been separated through electrolysis, when they recombine this produces a potential difference/ external current
-do not get less efficient the longer they run
-can be a source of drinkable water
-run on hydrogen which is an explosive gas making it difficult to store
-produce a fairly low p.d so several are needed