Orgo 2-Aromatic Compounds Ch 17
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
41 Terms
Nomenclature of Aromatic Compounds
1) parent chain
2) functional groups
3) substituents(branching)
4) numerical location
you can use ortho/para/meta instead of numbers
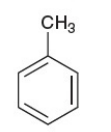
toluene
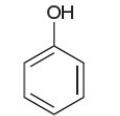
phenol
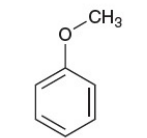
anisole
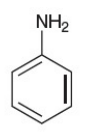
aniline
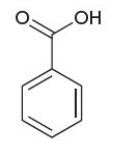
benzoic acid
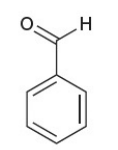
benzaldehyde
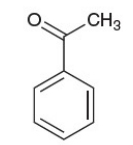
acetophenone
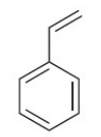
styrene
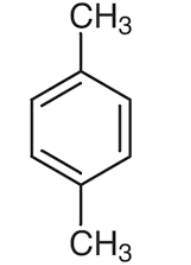
xylene
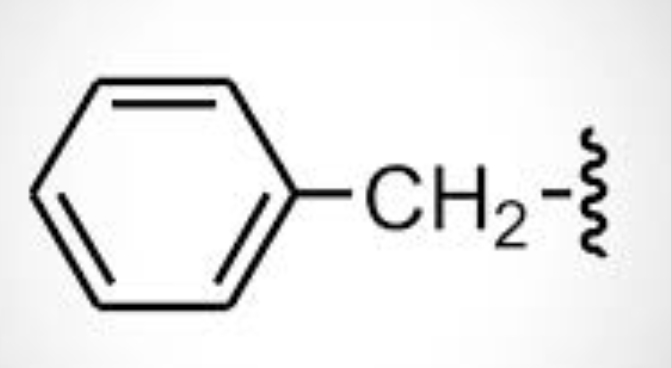
benzyl
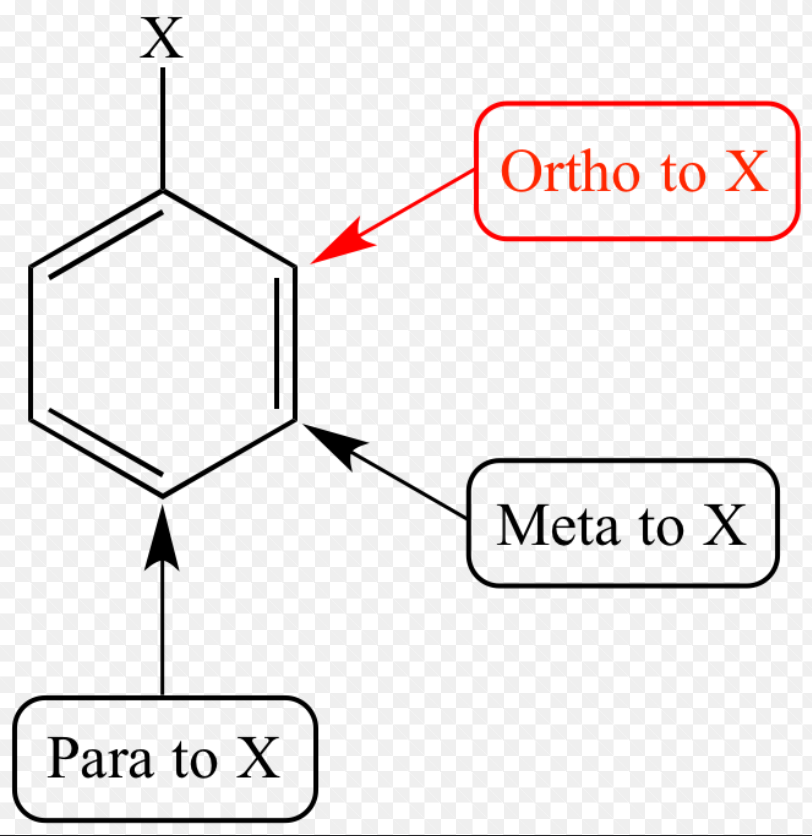
ortho
1,2
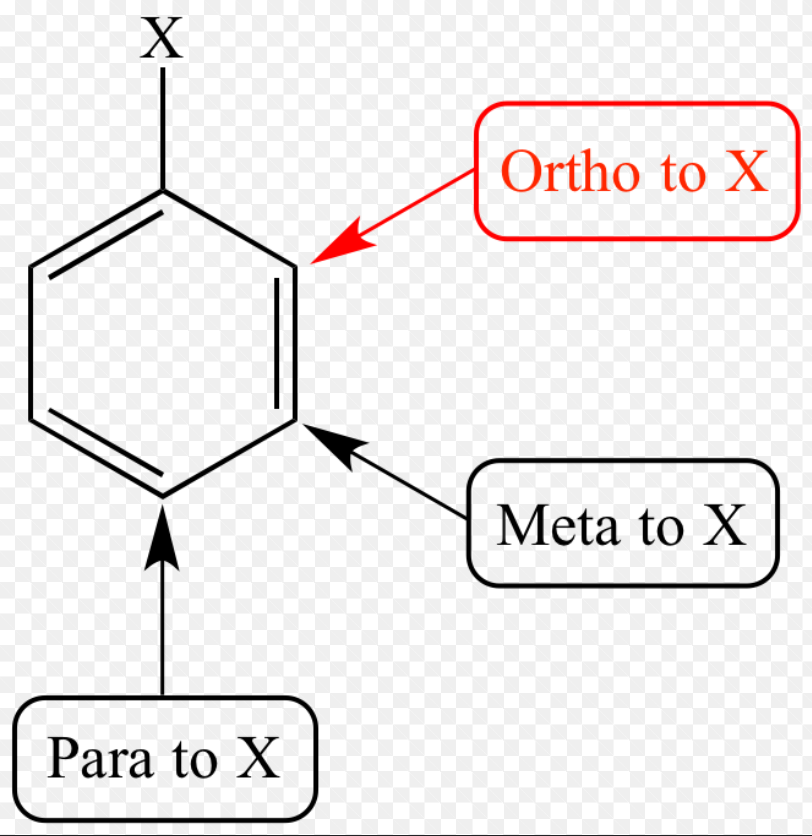
meta
1,3
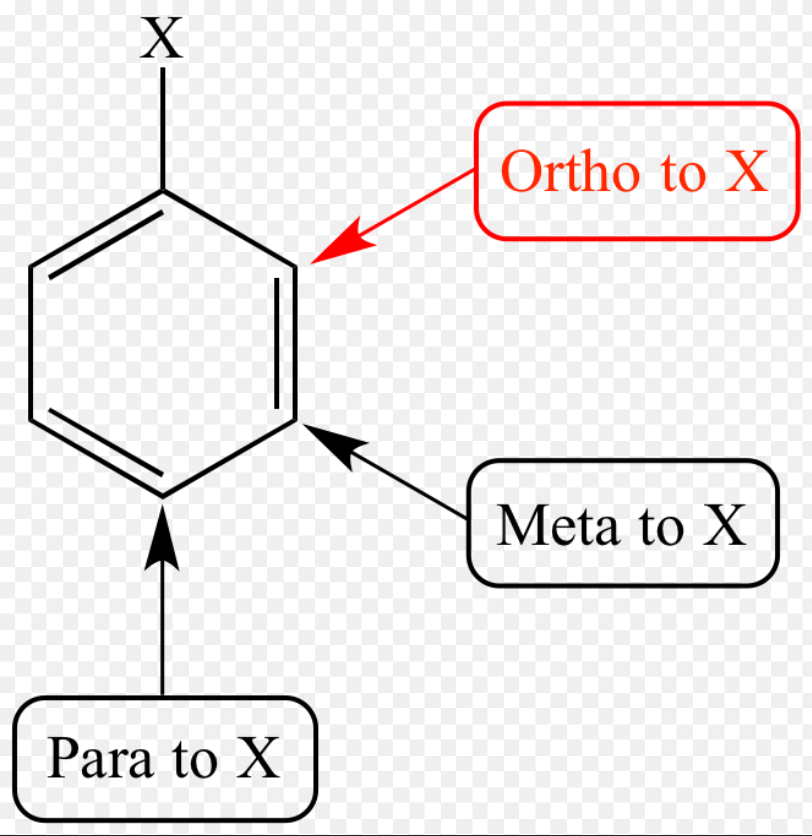
para
1,4
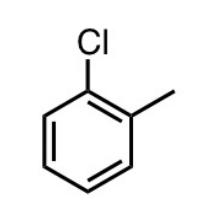
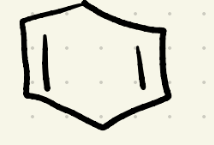
what’s the name for this?
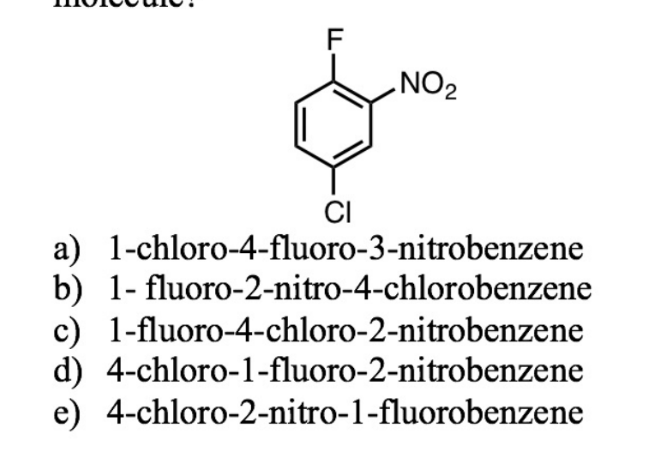
what’s the best name for this?
d
Huckles rule
determines aromatic, anti, or non aromatic (4n+2=2,6,10)
Frost circles
1) draw a polygon with one corner facing downwards
2) draw MO’s on all corners
3) Draw line that splits in the middle
4) above the line is antibonding, below is bonding, on line is non-bonding
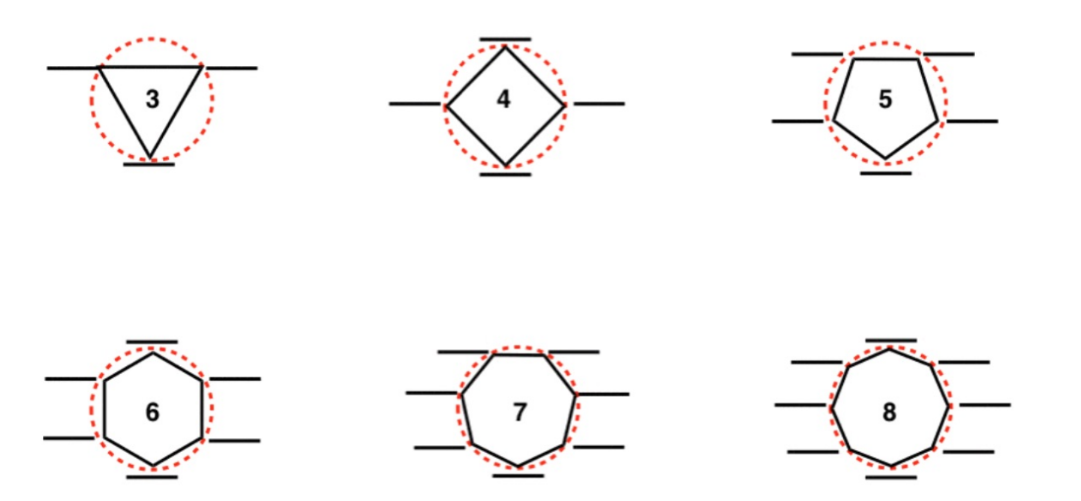
Requirements for aromaticity
1)cyclic
2) fully conjugated (pi bond skips sides)
3) planar (6 elements or less)
4) Huckles rule (4n+2=even number)
failure of aromaticty test (doesn’t meet 1 or more of the crieteria)
non aromatic
Meets all aromaticity conditions but only has 4n pi electrons
anti aromatic
what’s the stability flow?
aromatic>non-aromatic>anti-aromatic
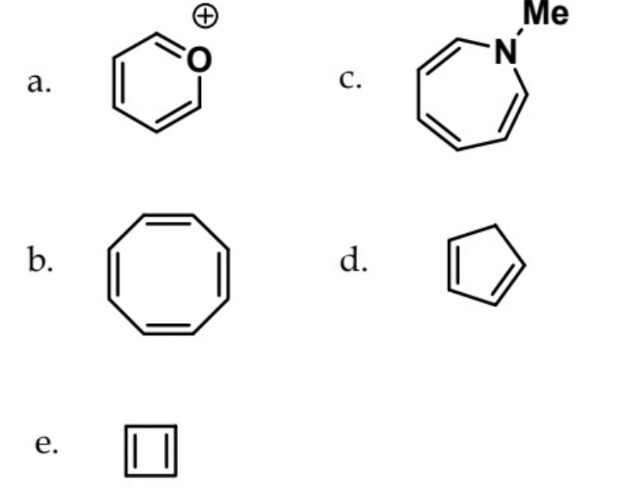
which one is aromatic
a
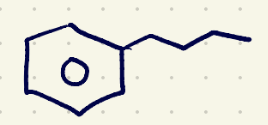
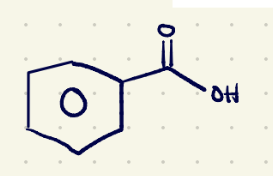
(1) oxidation reagents 1(must have 1 hydrogen at the benzylic position)
Jones reagent (Na2Cr2O7) and H2SO4,H2
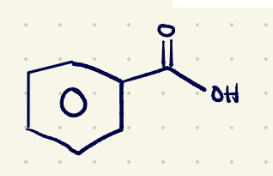
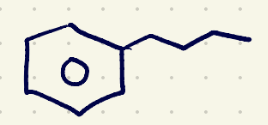
(1) oxidation reagents 2 (must have 1 hydrogen at the benzylic position)
KMnO4/H2O,Heat……..H3O+(quenching)
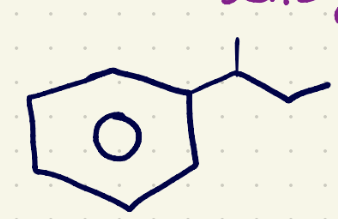
(2) Free Radical bromination reagents
NBS/Heat
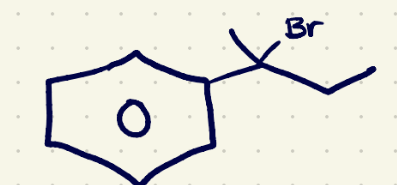
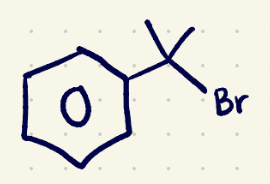
(3) Substitution Reaction reagents 1
H3O/(sn2)
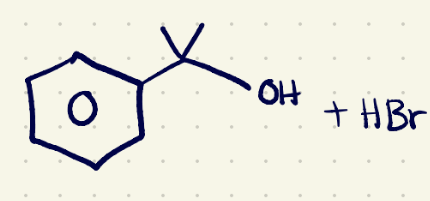
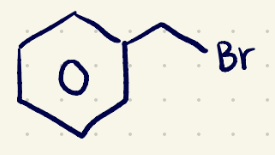
(3) Substitution Reaction reagents 2
NaOH/(sn2)
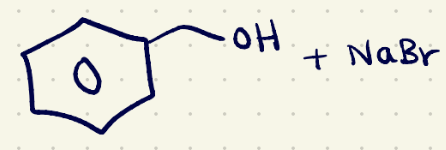
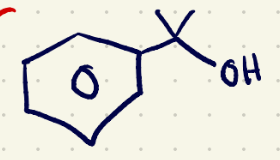
(3) Elimination rxn reagents 1
conc H2SO4/ (e1)
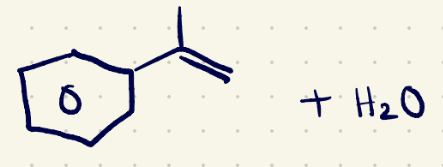
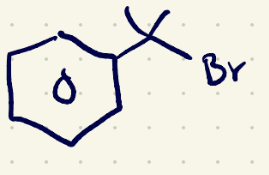
(3) Elimination rxn reagents 2
NaOEt/(e2)
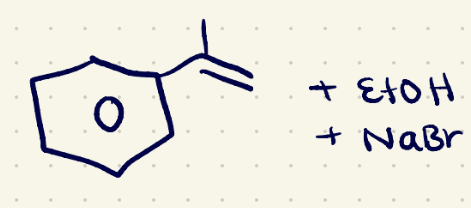
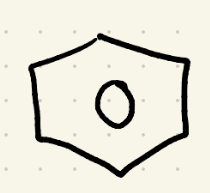
Reduction (Hydrogenation 1) reagents
3H2, Ni/100atm 150C
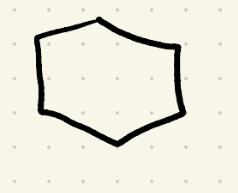
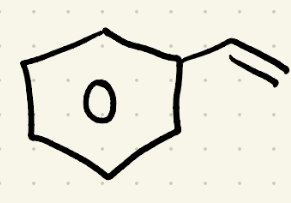
Reduction (Hydrogenation 2) reagents
H2, Pt/ 2atm 25C
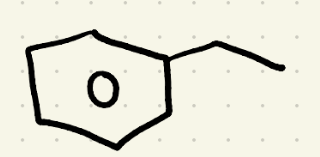
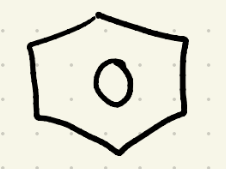
Birch reduction
Na, CH3OH/NH3
(carbons attached to alkyl groups won’t be reduced only when attached to electron-withdrawing groups.)



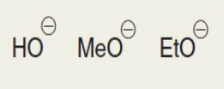

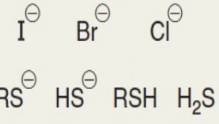


SN2 rxns
strong nu, unsubstituted, backside attack
SN1 reactions
neutral nu, highly substituted
E2 rxn
strong nu, highly subst, regioselective, stereospecific
E1 rxn
weak nu, highly substi, no anti-coplanar