Pathophysiology II Exam 4 - CARDIO pt. 2
1/167
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
168 Terms
progression of arterial diseases
coronary artery disease -> myocardial ischemia -> acute coronary syndromes
coronary artery disease
any vascular disorder that narrows or occludes the coronary arteries
coronary artery disease can result in
an imbalance between coronary supply of blood and myocardial demand for oxygen and nutrients causing ischemia or irreversible infarction
most common cause of coronary artery disease
atherosclerosis
non-modifiable risk factors for coronary artery disease
- advanced age
- family history
- male gender or being a woman after menopause
modifiable risk factors for coronary artery disease
- dyslipidemia
- hypertension results in endothelial injury and increase in myocardial demand
- cigarette smoking results in vasoconstriction and an increase in LDL/decrease in HDL
- diabetes and insulin resistance results in endothelial damage and thickening of the vessel wall
- obesity and/or sedentary lifestyle
- atherogenic diet
metabolic syndrome is characterized by
obesity, dyslipidemia, and hypertension
dyslipidemia
abnormal concentrations of serum lipoproteins that has a strong association with coronary artery disease
patho of dyslipidemia
- dietary fat packaged into chylomicrons for absorption in the small intestine
- chylomicrons function by transporting exogenous lipid from the intestine to the liver and the peripheral cells
- primarily contains triglycerides that may be removed and either stored by adipose tissue or used by muscle as an energy source
- remnant contains cholesterol, which is then taken up by the liver
increased LDL as an indicator of coronary risk
plays a role in endothelial injury, inflammation, and immune responses that are important in atherogenesis
decreased HDL as an indicator of coronary risk
responsible for "reverse cholesterol transport," which returns excess cholesterol from the tissues to the liver
other indicators of coronary risk
- elevated serum VLDL (triglycerides)
- increased lipoprotein (a)
non-traditional risk factors for coronary artery disease
- markers of inflammation, ischemia, and thrombosis (C-reactive protein)
- chronic kidney disease
- adipokines and other obesity complications
- medications
- microbiome
- air pollution and ionizing radiation
- coronary artery calcification, carotid wall thickness
transient myocardial ischemia
develops if the supply of coronary blood cannot meet the demand of the myocardium for oxygen and nutrients, but perfusion is restored before there is permanent damage
how long does it take for ischemia to develop?
within 10 seconds
duration of myocardial oxygen deficit that distinguishes transient ischemia from permanent ischemia
20 minutes
stable angina
recurrent, predictable chest pain
patho of stable angina
gradual luminal narrowing and hardening of the arterial walls causing the affected vessels to not be able to dilate in response to increased myocardial demand associated with physical exertion or emotional stress
characteristics of stable angina
- reproducible (i.e., this happens every time I mow my yard, etc.)
- with rest, myocardial blood flow is restored and no necrosis of myocardial cells results
angina pectoris
chest pain caused by myocardial ischemia often characterized as transient and substernal discomfort
prinzmetal angina (variant)
causes unpredictable chest pain that often occurs at night during REM sleep
patho of prinzmetal angina
vasospasm may result from decreased vagal activity, hyperactivity of the SNS, and decreased nitric oxide activity
silent ischemia and mental stress (induced)
causes no detectable symptoms (i.e., fatigue, dyspnea, or feeling of unease)
patho of silent ischemia and mental stress (induced)
mental stress can result in the release of catecholamines and increase in HR, BP, and vascular resistance
acute coronary syndromes
sudden coronary obstruction due to thrombus formation over a ruptured atherosclerotic plaque (i.e., unstable angina, MI)
unstable angina
reversible myocardial ischemia and a harbinger of impending infarction
characteristics of unstable angina
transient episodes of thrombotic vessel occlusion and vasoconstriction occur at the site of plaque damage with a return of perfusion before significant myocardial necrosis occurs
myocardial infarction (MI)
prolonged ischemia causes irreversible damage to the heart muscle (myocyte necrosis)
characteristics of myocardial infarction
- subendocardial vs. transmural
- cellular injury leading to cellular death
- structural and functional changes
- repair
structural and functional changes seen following myocardial infarction
- myocardial stunning
- hibernating myocardium
- myocardial remodeling
myocardial stunning
the temporary loss of contractile function that persists for hours to days after perfusion has been restored
hibernating myocardium
tissue, that is persistently ischemic, undergoes metabolic adaptation to prolong myocyte survival
myocardial remodeling
process that occurs in the myocardium after an MI
repair following an MI
degradation of damaged cells, proliferation of fibroblasts, and synthesis of scar tissue
overall effects of angiotensin II following an MI
involved in myocardial remodeling as it causes myocyte hypertrophy, scarring, and loss of contractile function in the areas of the heart distant from the site of the infarction
systemic angiotensin II effects following an MI
peripheral vasoconstriction and fluid retention results in myocardial work increasing thus the effects of the loss of myocyte contractility are exacerbated
local angiotensin II effects following an MI
growth factor for vascular smooth muscle cells, myocytes, and cardiac fibroblasts promotes catecholamine release and causes coronary artery spasms
clinical manifestations of MI
- infarcted myocardium is surrounded by a zone of hypoxic injury, which may progress to necrosis or return to normal; adjacent to this zone is a zone of reversible ischemia
- sudden severe chest pain
- ECG changes (STEMI or NSTEMI)
- troponin I elevates within 2-4 hours
- creatine phosphokinase-MB, LDH
- hyperglycemia
- leukocytosis, elevated CRP seein in the repair phase
what is the most specific clinical manifestation for an MI?
elevated troponin I levels
complications of MI
- dysrhythmias
- heart failure
- cardiogenic shock
- pericarditis
- ventricular aneurysm
acute pericarditis
acute inflammation of the pericardium
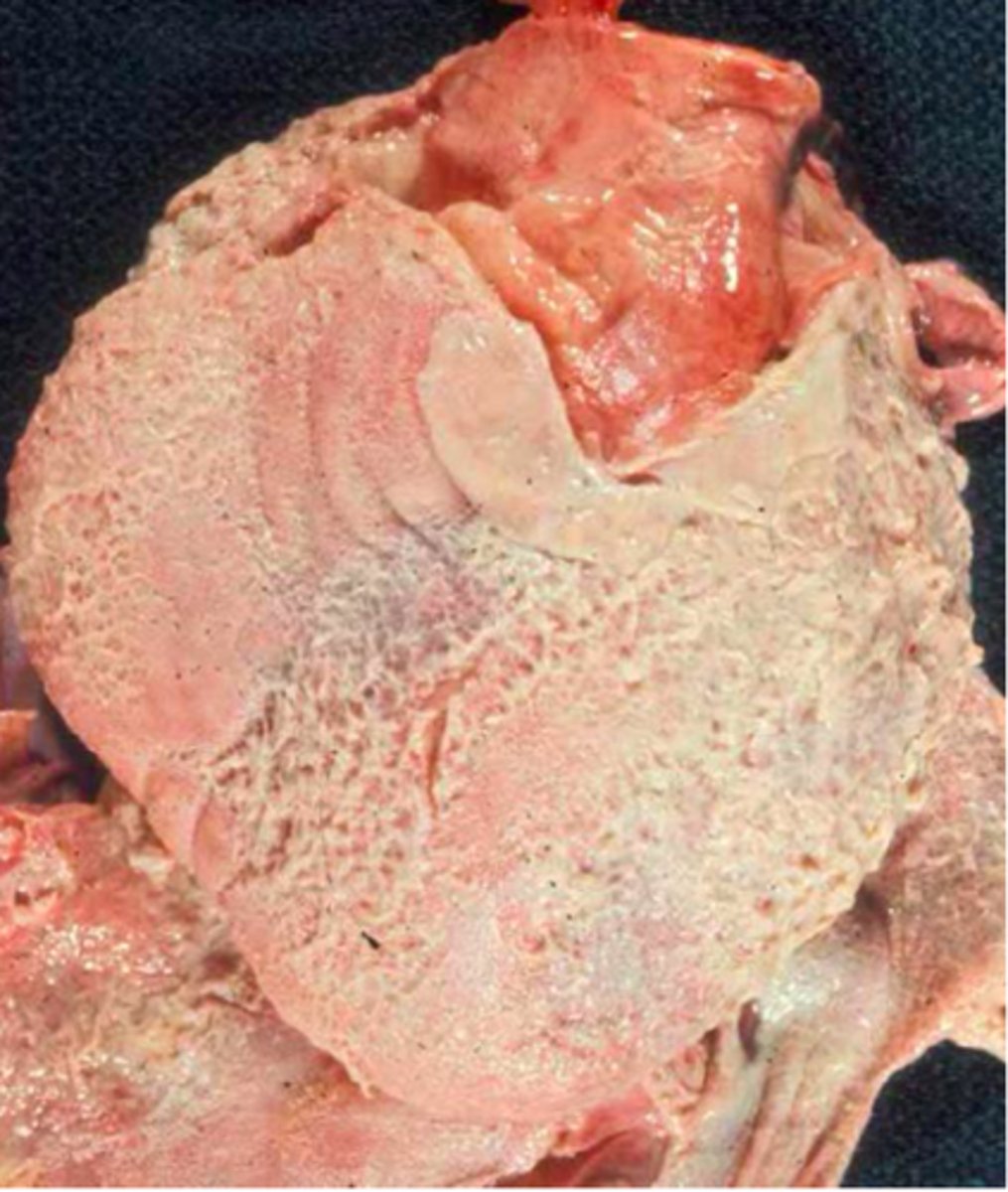
patho of acute pericarditis
most often idiopathic but can be viral
symptoms of acute pericarditis
- fever, myalgia, malaise
- chest pain with movement or lying down
- sinus tachy
- friction rub (highly specific)
pericardial effusion
accumulation of fluid in the pericardial cavity
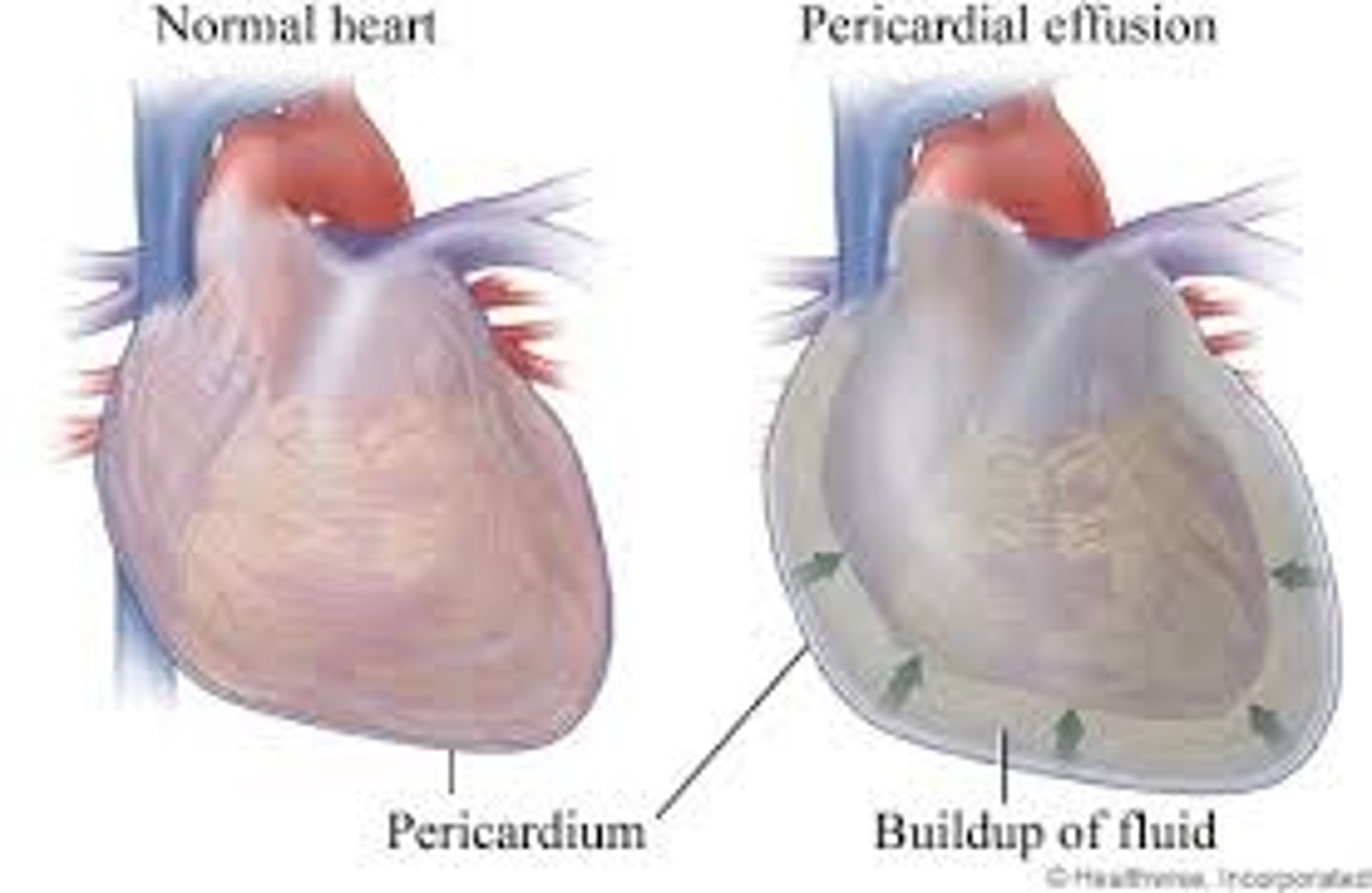
transudative pericardial effusion
- extravascular fluid, low in protein, non-inflammatory
- could be due to heart failure or overhydration
exudative pericardial effusion
- direct irritation pericardium
- inflammation, infection, malignancy, autoimmune
complications of pericardial effusion
- if fluid buildup develops rapidly, tamponade can occur
- if fluid buildup develops slowly, the pericardial sac accommodates larger amounts of fluid without compression
cardiac tamponade
fluid accumulation in the pericardium that compresses the heart
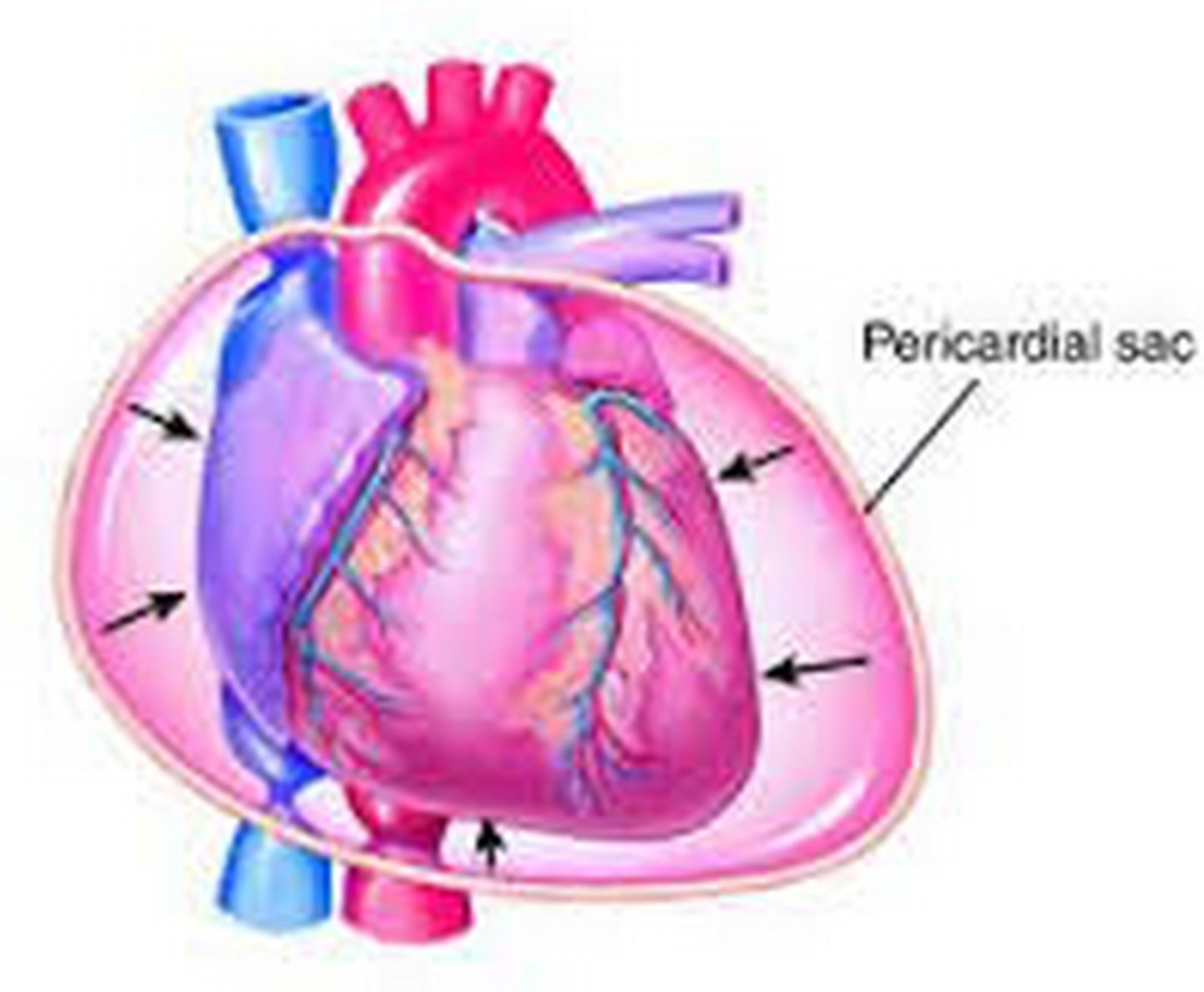
dangers of cardiac tamponade
when pressure exerted by the fluid equals diastolic pressure in the chambers (usually the right side first)
symptoms of cardiac tamponade
- JVD
- reduced cardiac output
- pulsus paradoxus
- water bottle configuration on CXR
pulsus paradoxus
arterial BP during expiration exceeds the pressure during inspiration by more than 10 mmHg
constrictive (restrictive) pericarditis
fibrous scarring with occasional calcification of the pericardium causes the visceral and parietal pericardial layers to adhere and encase the heart in a rigid shell
clinical manifestations of constrictive (restrictive) pericarditis
exercise intolerance, dyspnea on exertion, fatigue, and anorexia
cardiomyopathies
effects of neurohumoral responses to ischemic heart disease or hypertension on the heart muscle cause remodeling
patho of cardiomyopathies
many cases are idiopathic
dilated cardiomyopathy
impaired systolic function leading to increases in intracardiac volume, ventricular dilation, and systolic heart failure (thin walls)
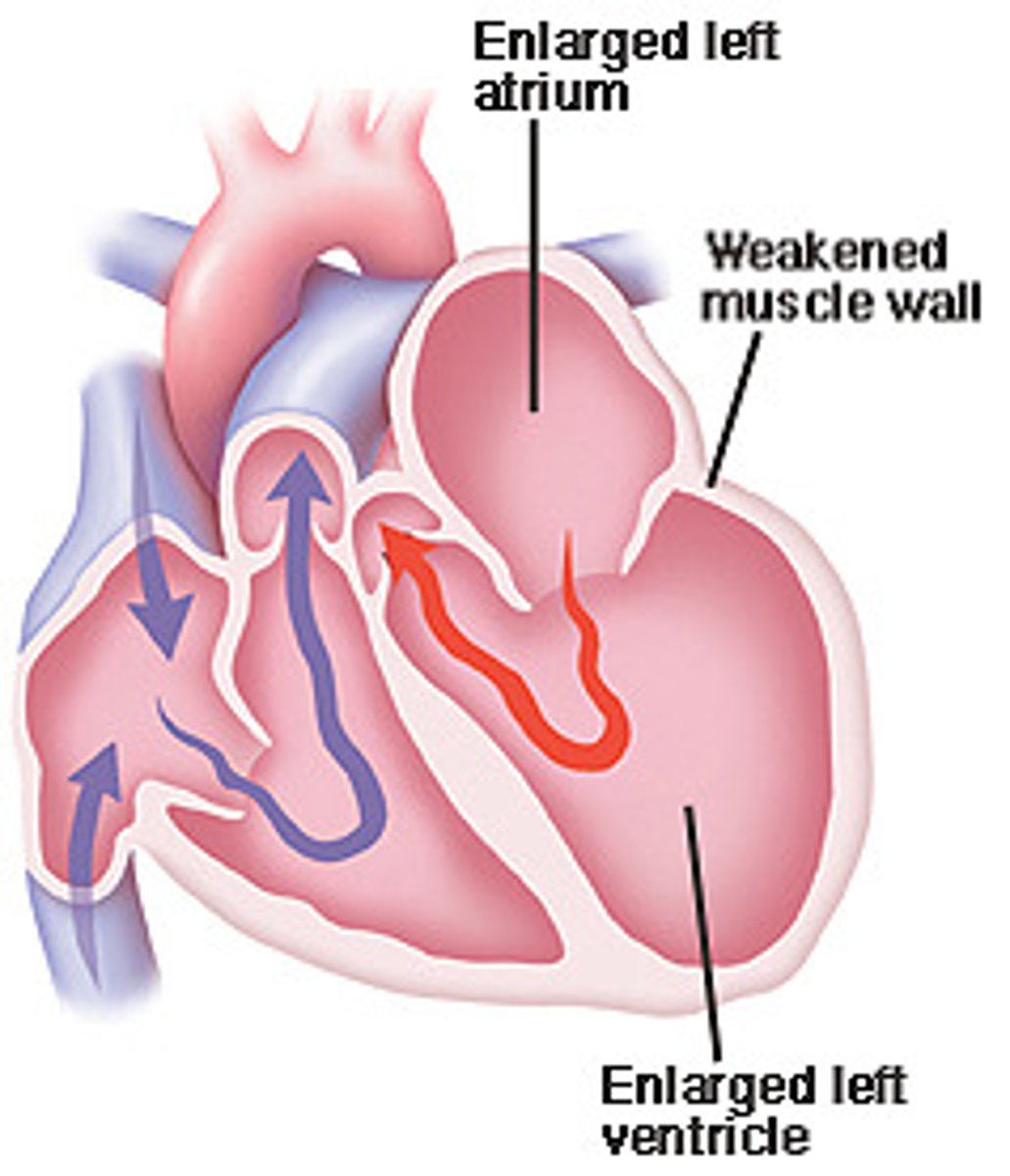
causes of dilated cardiomyopathy
- ischemic heart disease
- valvular disease
- diabetes
- alcohol
- drug toxicity
- renal failure
- hyperthyroidism
- deficiencies of niacin, vitamin D, and selenium
- infection
clinical manifestations of dilated cardiomyopathy
dyspnea, fatigue, and pedal edema
hypertrophic cardiomyopathy
- thickening of the septal wall
- most common inherited heart defect
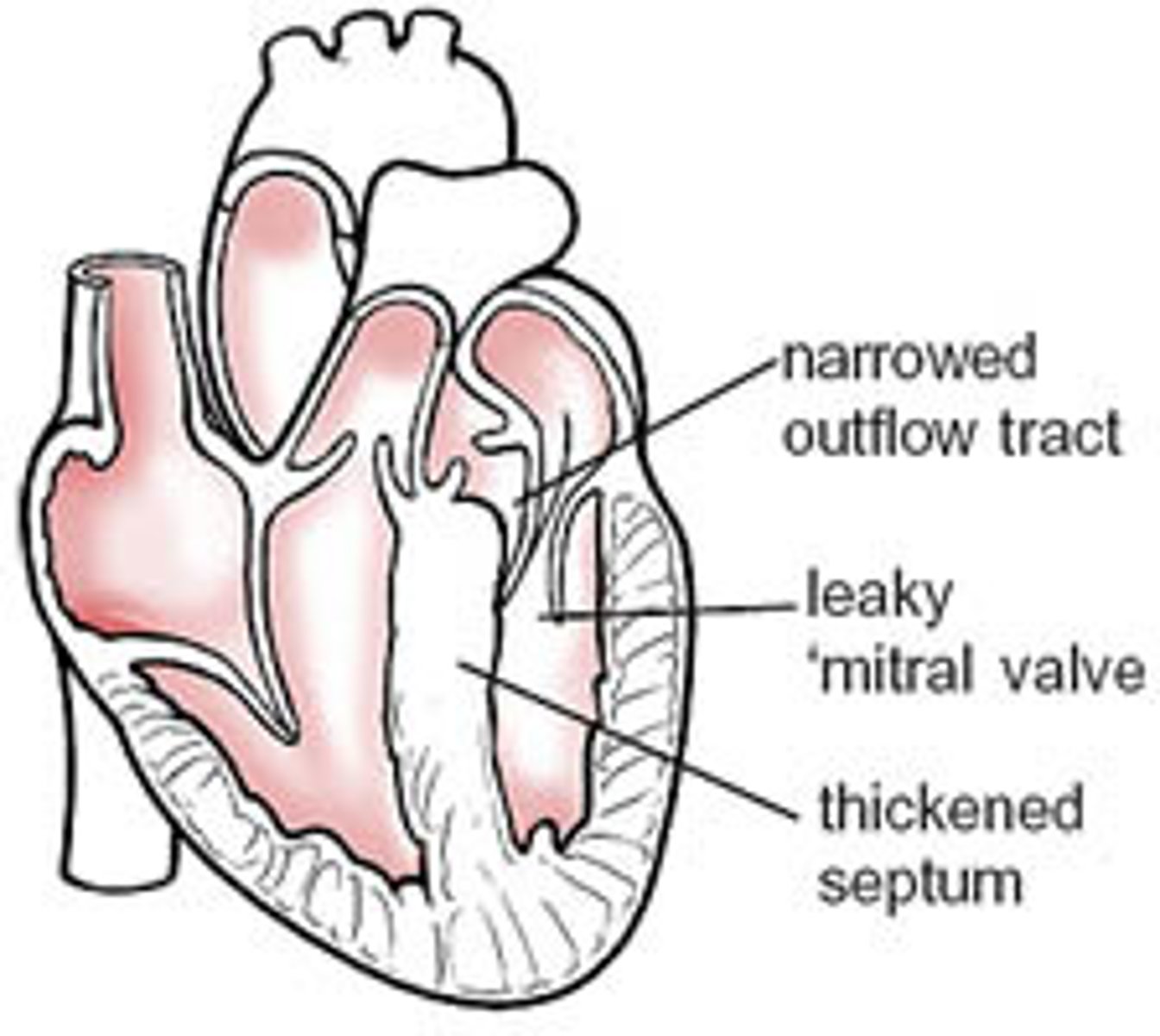
effects of hypertrophic cardiomyopathy
obstruction of left ventricular outflow tract (more apparent whenever heart rate increases)
clinical manifestations of hypertrophic cardiomyopathy
angina, syncope, palpitations, left heart failure, MI symptoms
hypertensive (valvular hypertrophic) cardiomyopathy
hypertrophy of the myocytes in an attempt to compensate for increased myocardial workload
patho of hypertensive (valvular hypertrophic) cardiomyopathy
long-term dysfunction of the myocytes develops over time; first diastolic dysfunction leading to systolic dysfunction of the ventricle
clinical manifestations of hypertensive (valvular hypertrophic) cardiomyopathy
- can be asymptomatic
- may complain of angina, syncope, dyspnea on exertion, and palpitations
restrictive cardiomyopathy
myocardium becomes rigid and noncompliant, impeded ventricular filling and raising filling pressures during diastole
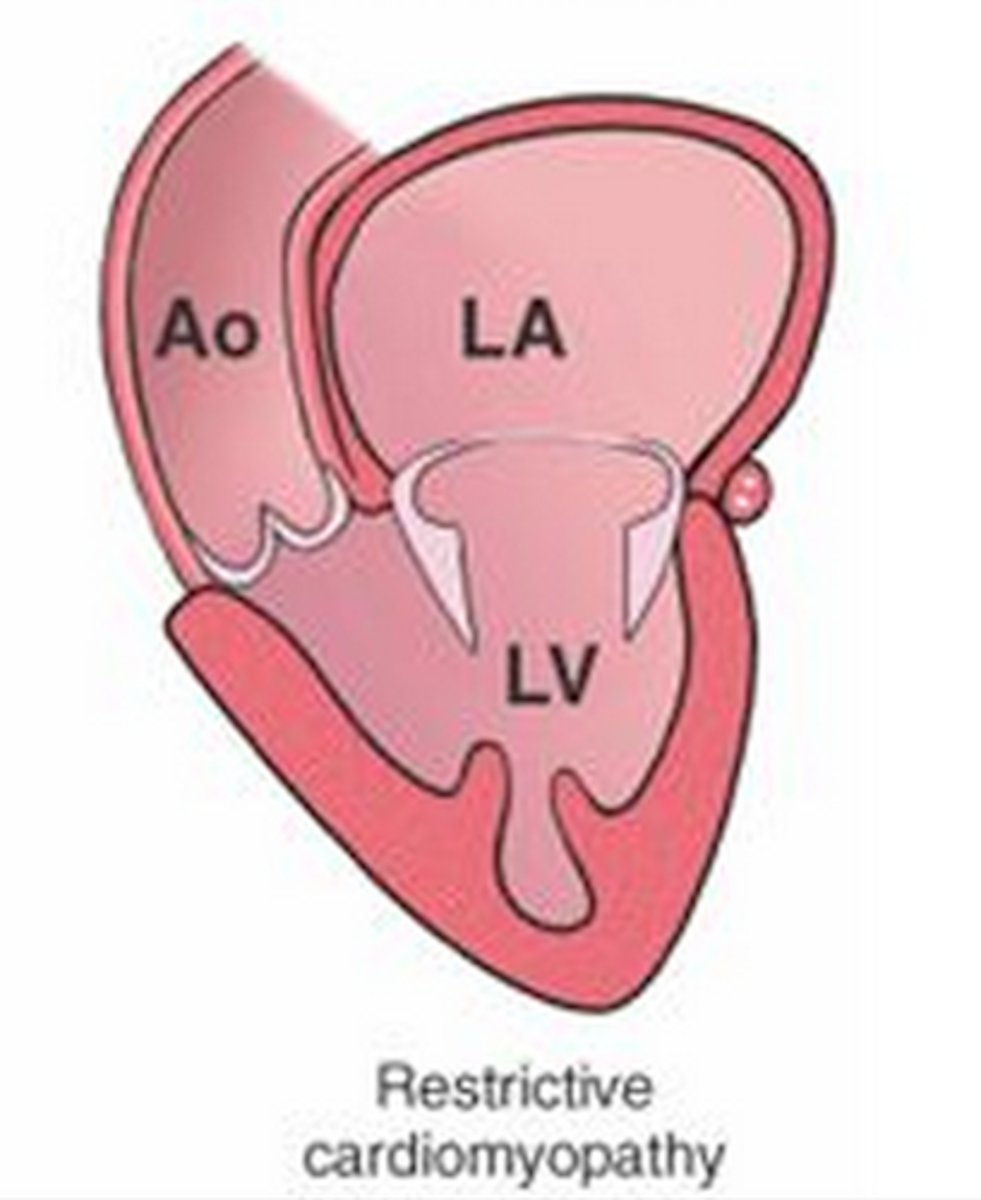
clinical manifestations of restrictive cardiomyopathy
right heart failure occurs with systemic venous congestion
common causes of restrictive cardiomyopathy
systemic disease (think scleroderma, amyloidosis, sarcoidosis, lymphoma, etc.)
valvular dysfunction
stimulates chamber dilation and/or myocardial hypertrophy
which side is affected more by valvular dysfunction?
the left heart (mitral and aortic valves) more commonly than the right heart (tricuspid and pulmonic valves)
possible causes of valvular dysfunction
- endocardial tissue
- congenital or acquired
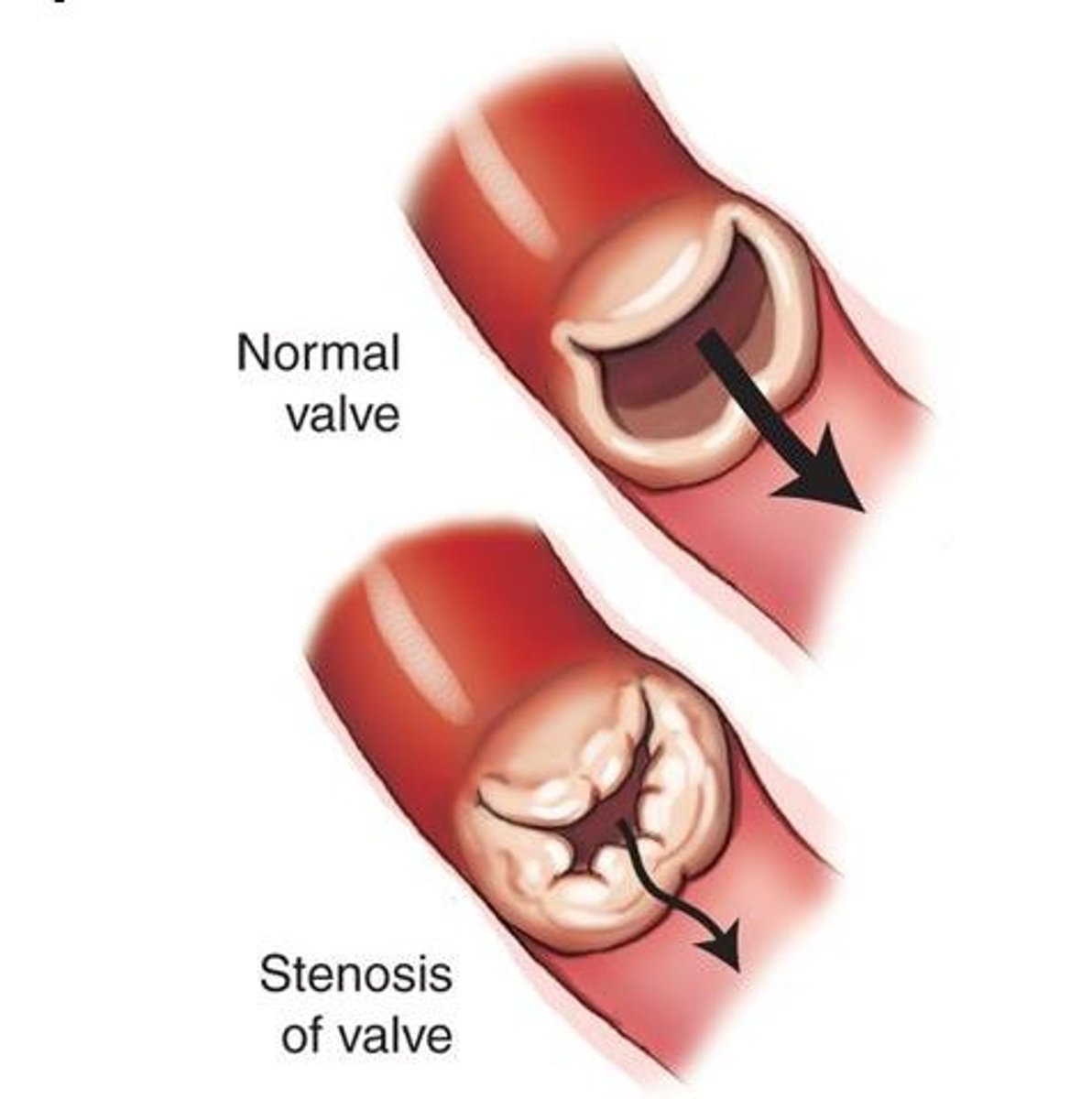
stenosis
- constricted, narrowed, impede forward flow
- increases workload, hypertrophy
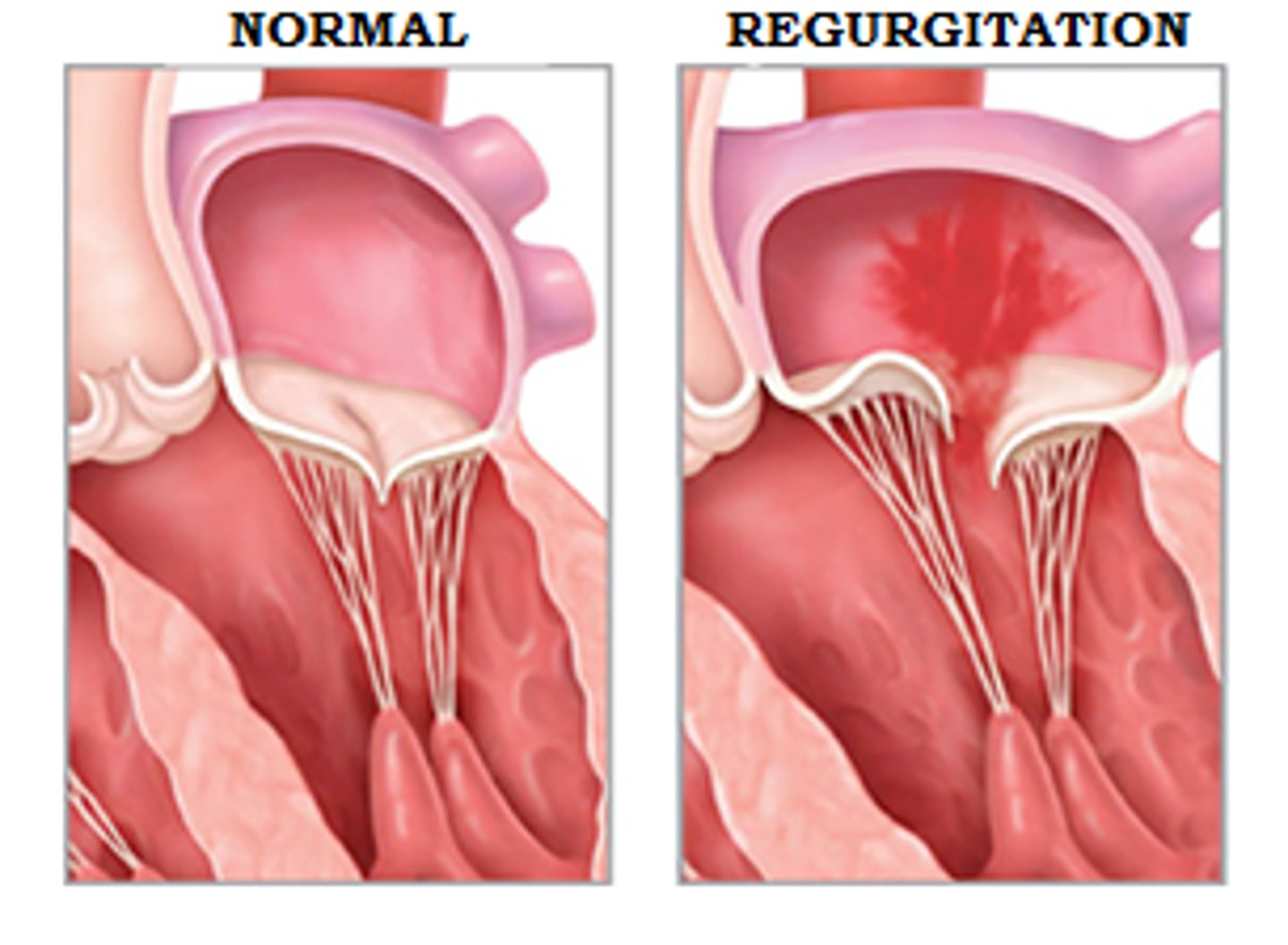
regurgitation
- fail to shut completely
- increases blood volume/workload, leads to dilation and hypertrophy
aortic stenosis
- most common valvular abnormality
- orifice of the aortic semilunar valve narrows causing diminished blood flow from the left ventricle into the aorta
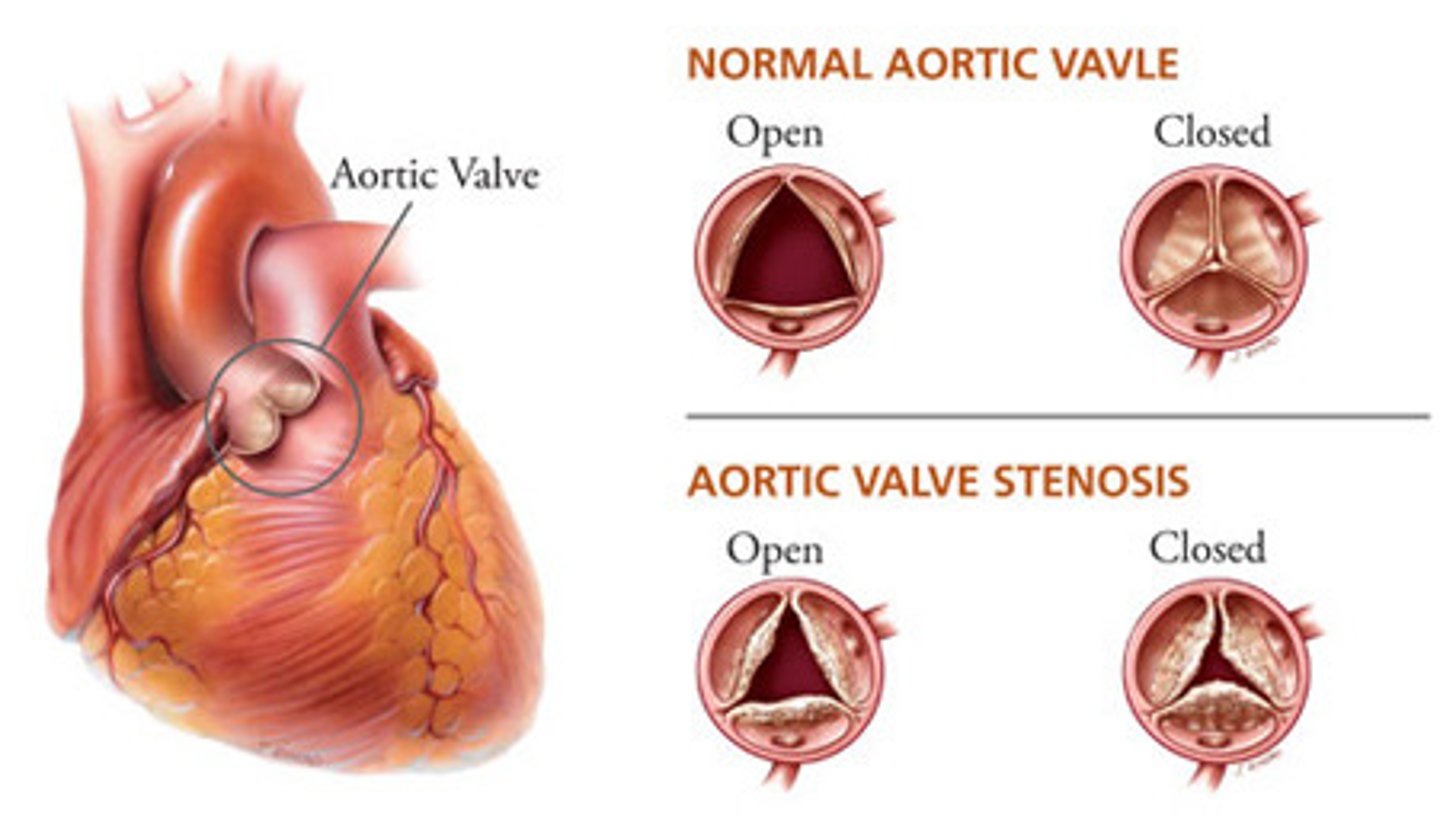
patho of aortic stenosis
- calcific degeneration related to aging (aortic sclerosis)
- congenital bicuspid valve
- inflammatory heart disease caused by rheumatic heart disease
clinical manifestations of aortic stenosis
angina, syncope, and dyspnea
mitral stenosis
impaired flow from left atrium to left ventricle
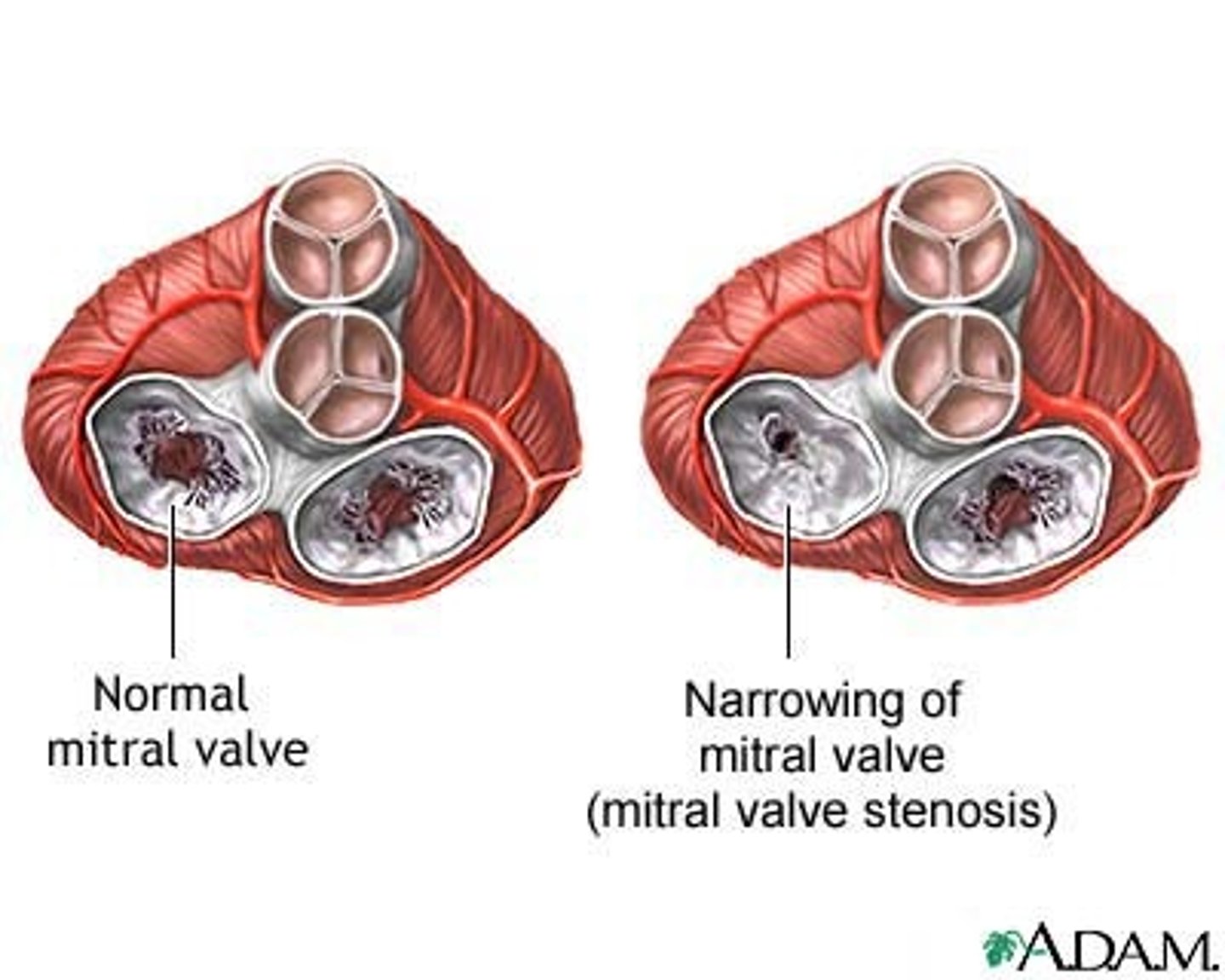
patho of mitral stenosis
most commonly caused by rheumatic disease (group A beta-hemolytic strep); autoimmune activation of lymphocytes and macrophages leads to inflammatory damage and scarring of valve leaflets
effects of mitral stenosis
- incomplete emptying of the left atrium
- chamber dilation and hypertrophy
- atrial arrhythmias
- decreased CO
aortic regurgitation
inability of the aortic valve leaflets to close properly during diastole
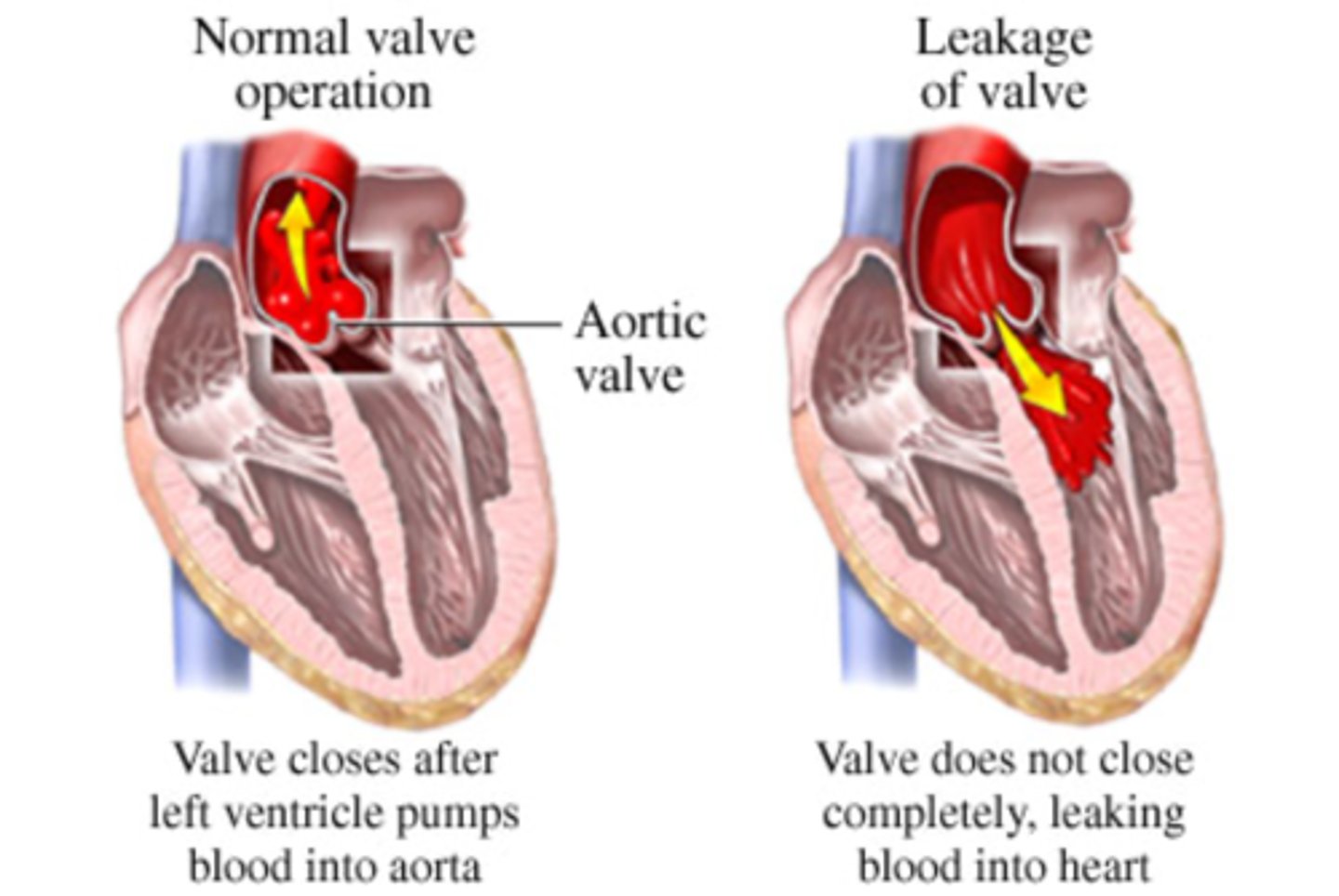
patho of aortic regurgitation
can be primary or secondary (i.e., from chronic HTN)
clinical manifestations of aortic regurgitation
widened pulse pressure as a result of increased stroke volume and diastolic backflow
mitral regurgitation
permits back-flow of blood from the left ventricle into the left atrium during systole
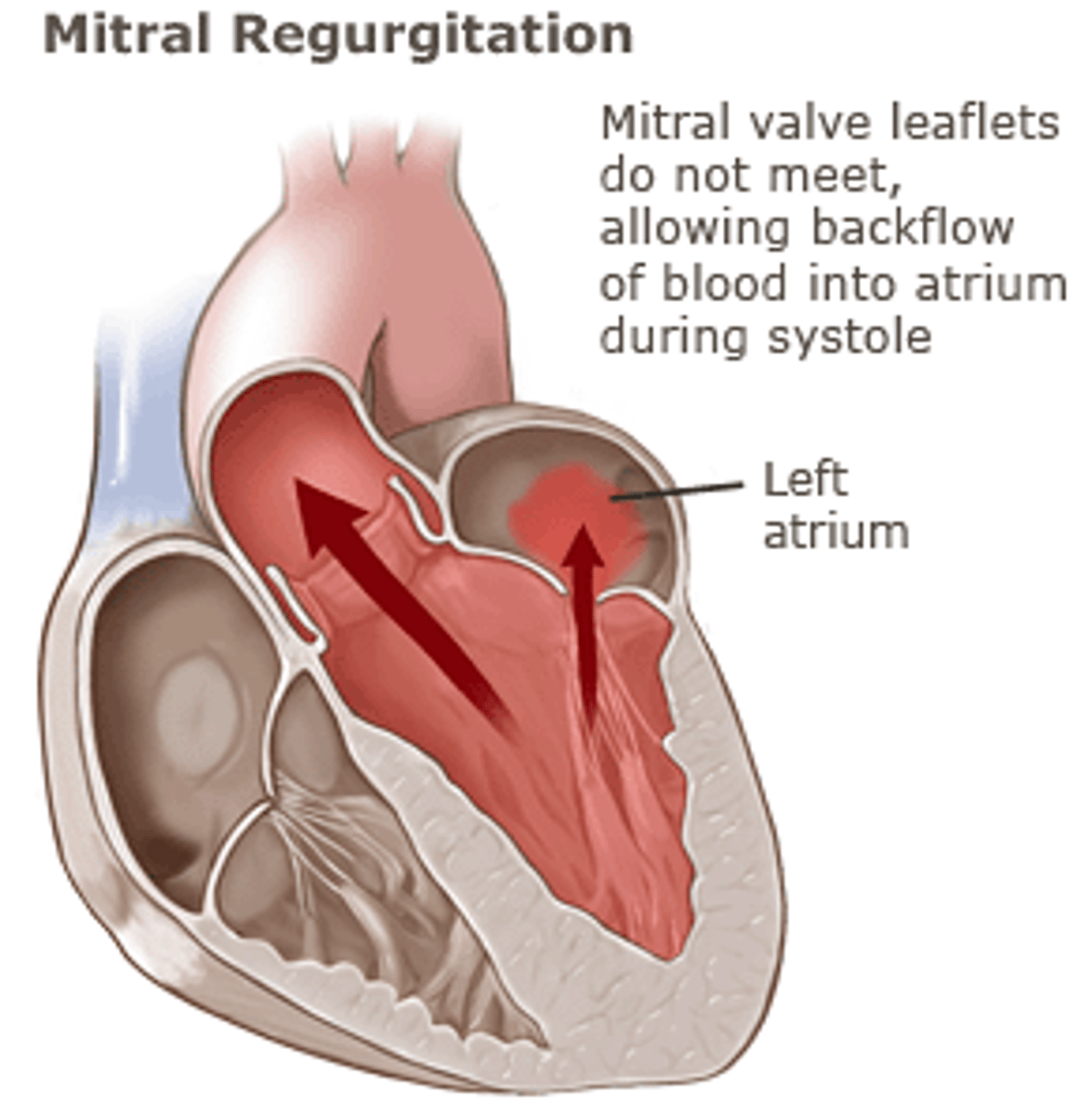
most common causes of mitral regurgitation
mitral valve prolapse, rheumatic heart disease, infective endocarditis, MI, connective tissue disease, dilated cardiomyopathy, etc.
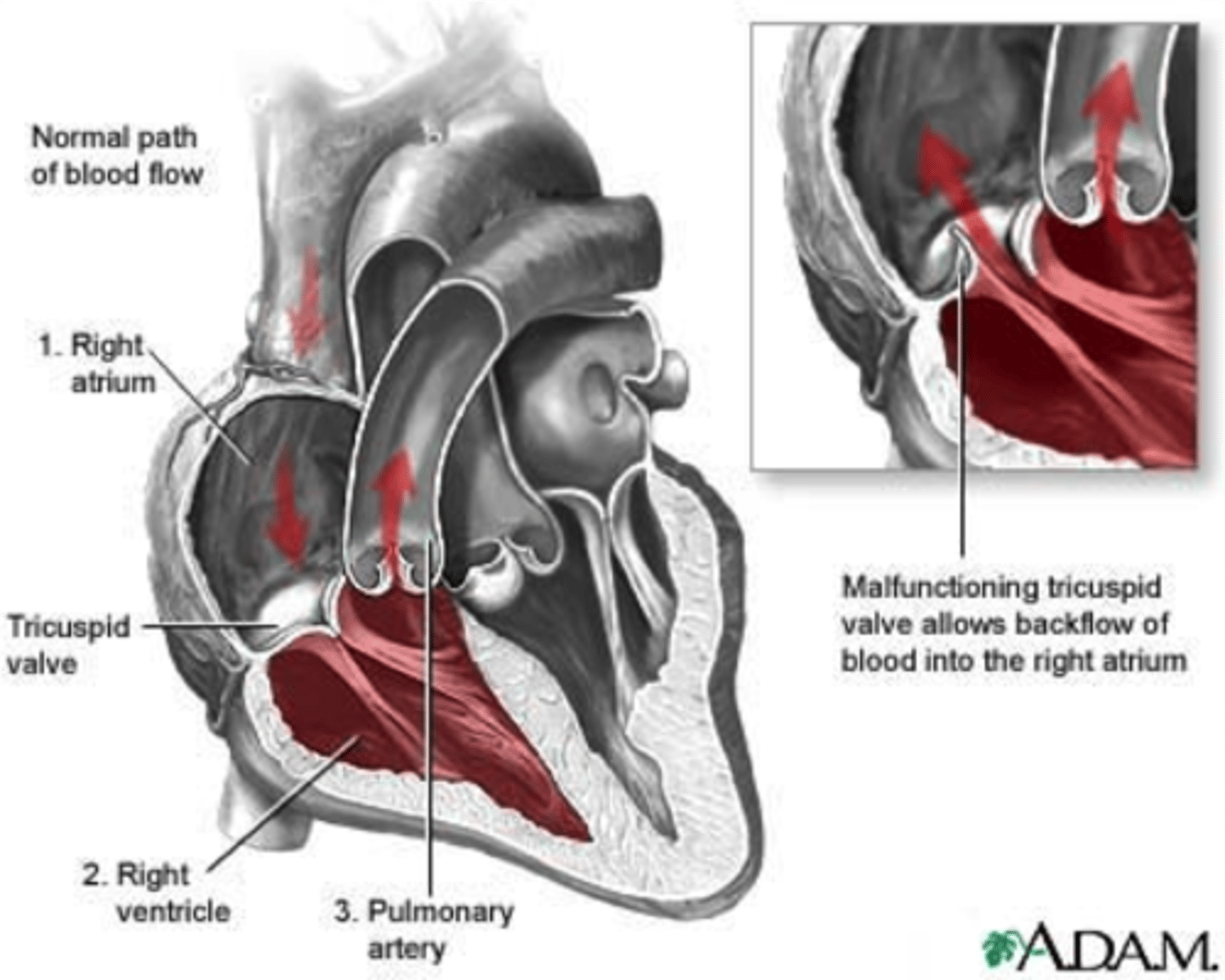
tricuspid regurgitation
leads to volume overload in the right atrium and ventricle, increased systemic venous blood pressure, and right heart failure
mitral valve prolapse syndrome
- most common valve disorder in the U.S. and more prevalent in young women
- anterior and posterior cusps of the mitral valve billow upward ("prolapse") into the atrium during systole
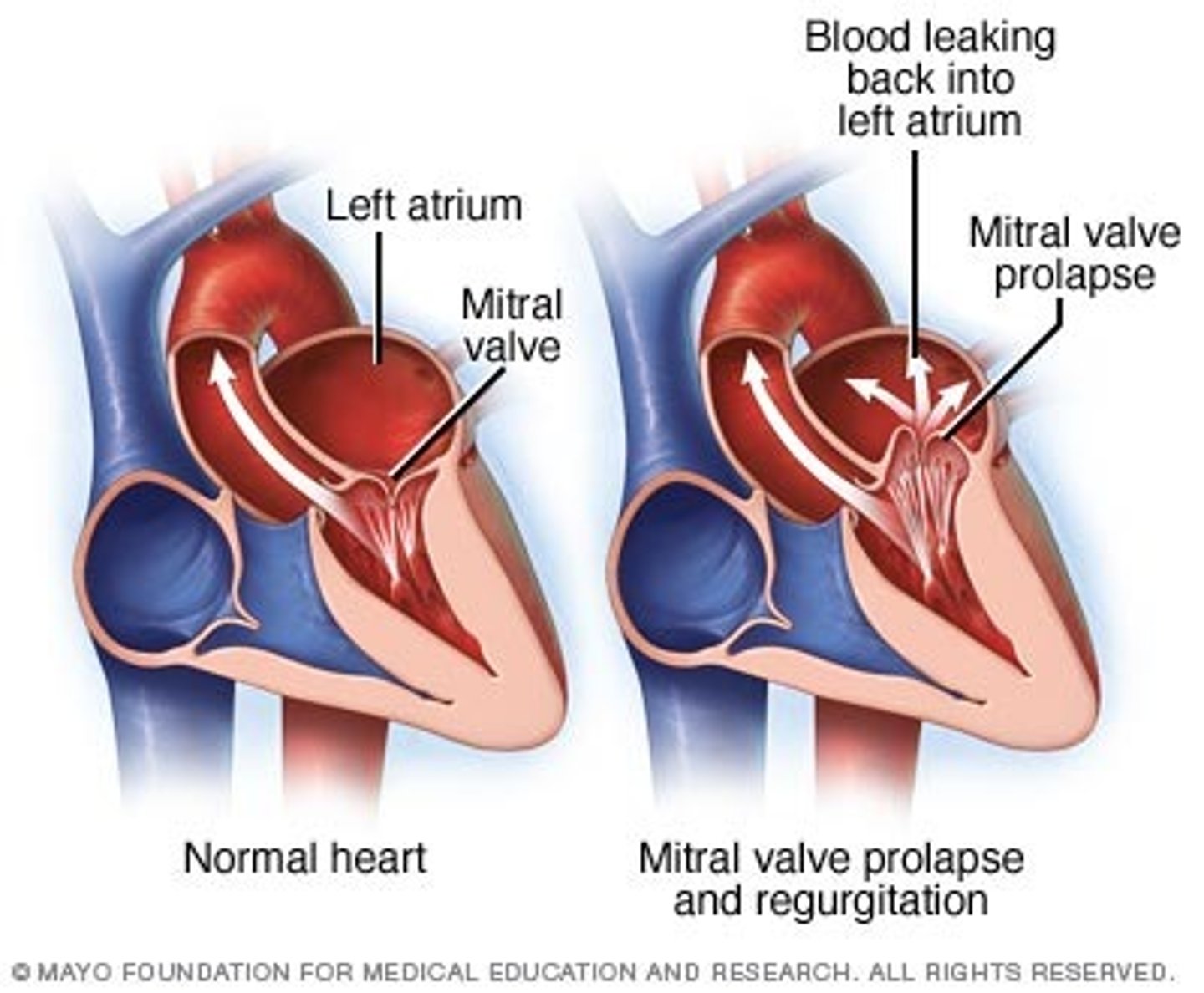
clinical manifestations of mitral valve prolapse syndrome
mostly asymptomatic but a murmur may be present
patho of mitral valve prolapse syndrome
associated with Marfan, Ehlers-danlos, osteogenesis imperfecta
rheumatic fever
a diffuse, inflammatory disease caused by a delayed immune response to infection by the group A beta-hemolytic streptococci
clinical manifestations of rheumatic fever
- a febrile illness
- fever, lymphadenopathy, arthralgia, nausea, vomiting, epistaxis
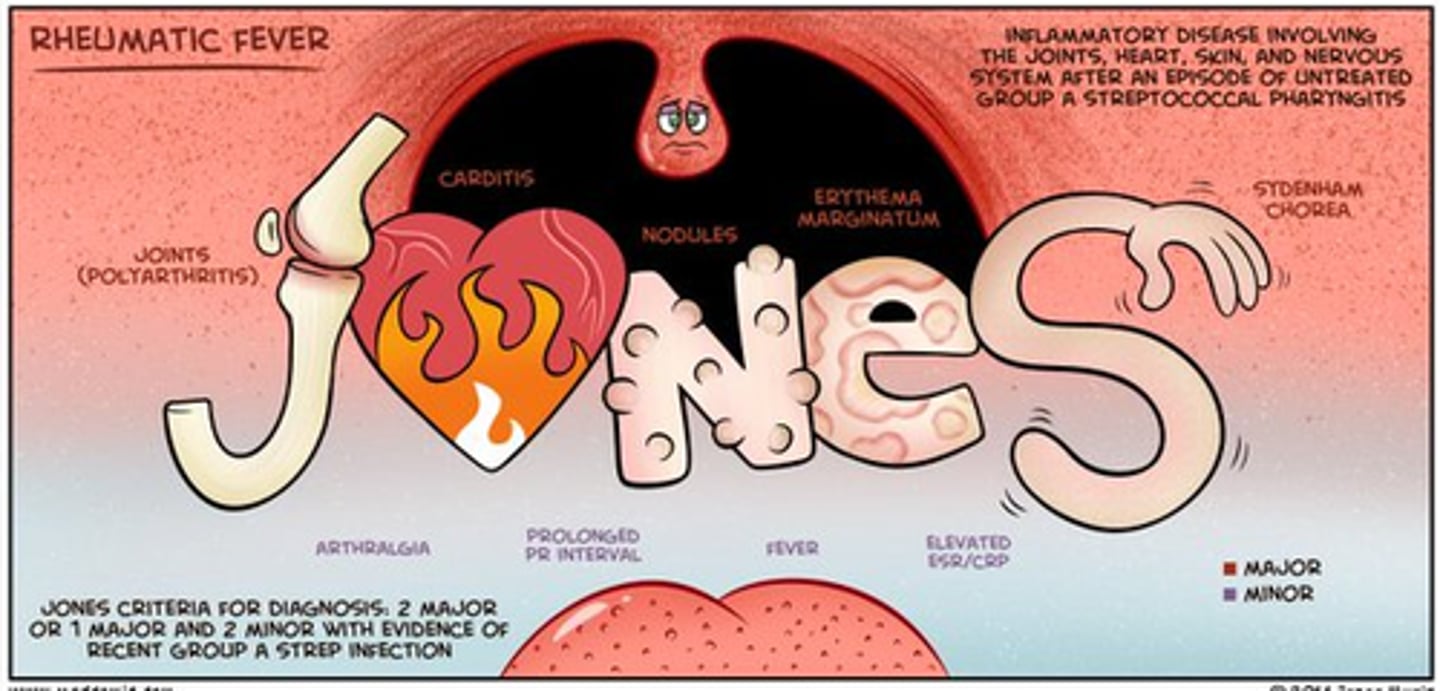
if left untreated, rheumatic fever causes
rheumatic heart disease
patho of rheumatic fever
- only as a sequel to pharyngeal infection by group A beta-hemolytic strep (skin strains do not have the same antigenic molecules)
- result of abnormal humoral and cell-mediated immune response to the M proteins on the microorganisms that cross react with normal tissues
rheumatic heart disease
heart disease caused by rheumatic fever in patients with a genetic susceptibility
clinical manifestations of rheumatic heart disease
- carditis
- polyarthritis
- subcutaneous nodules
- chorea
- erythema marginatum
carditis
- inflammation of the heart
- affects primarily the valves (swelling, erosion, and vegetation of platelets and fibrin deposited on chordae tedineae)
subcutaneous nodules
develop over bony prominences and along extensor tendons of elbows, wrists, knees, and ankles
chorea
sudden aimless involuntary movements (CNS)
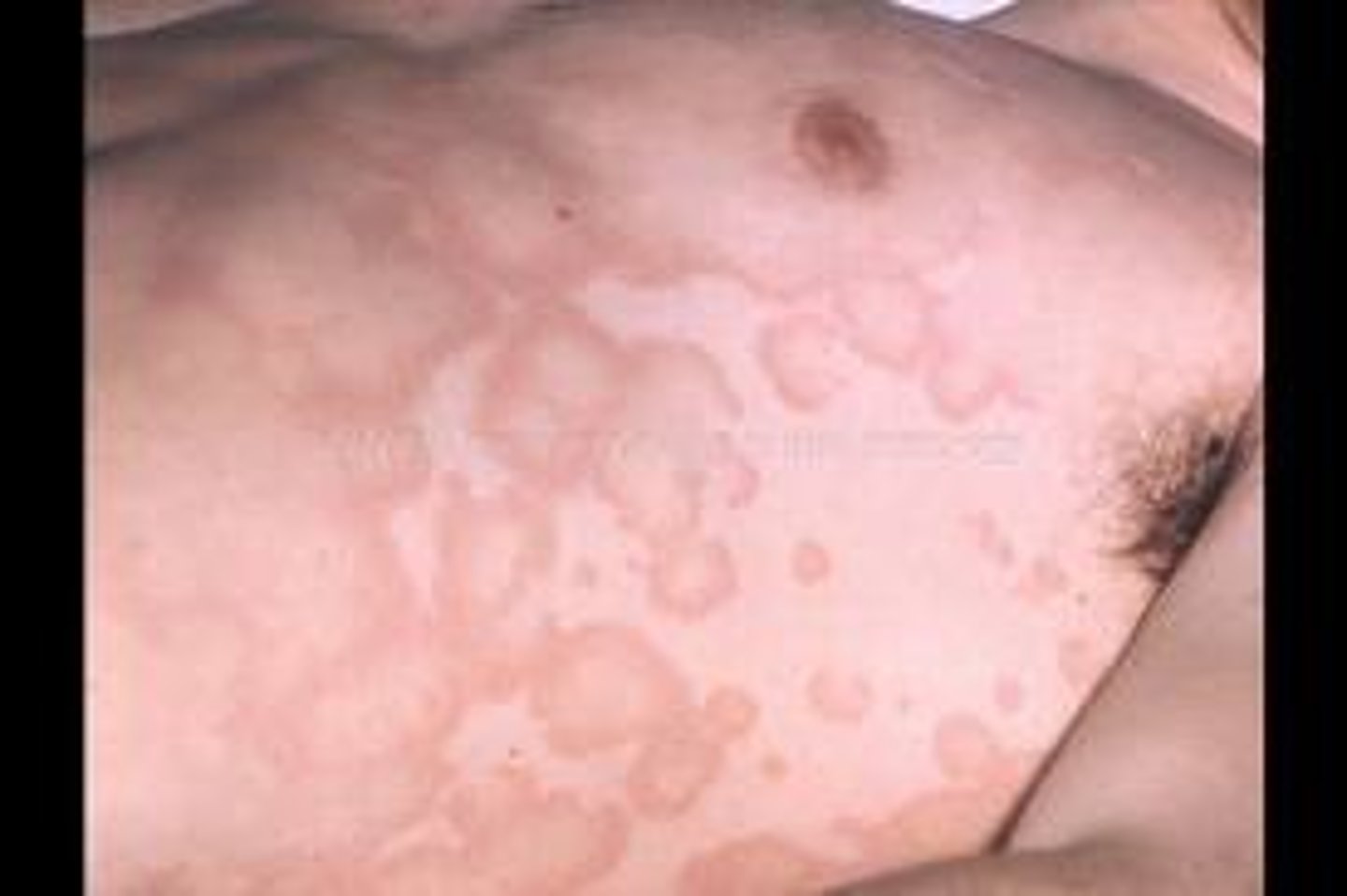
erythema marginatum
rash, pink, non-pruritic macules that never occur on the face or the hands
infective endocarditis
inflammation of the endocardium from infectious agents
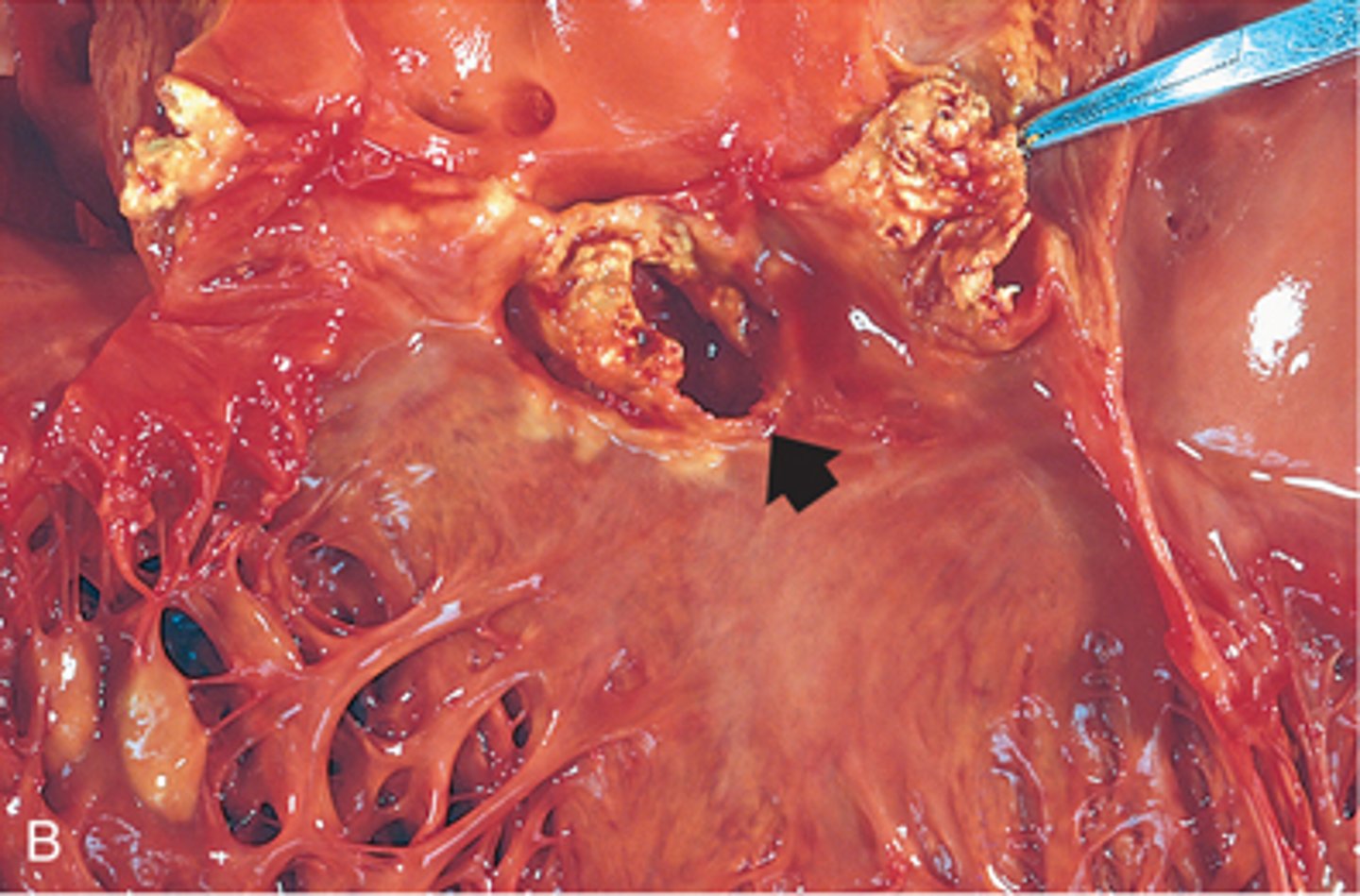
most common causes of infective endocarditis
bacteria (especially strep, staph, and enterococci)
patho of infective endocarditis
- endocardial damage due to trauma, congenital/valvular, present of prosthetic valve
- bloodborne microorganism adherence
- formation of infective endocardial vegetations