BMS 310 Test 1
1/153
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
154 Terms
Metabolism
Anabolism and Catabolism together
Catabolism-breaking
Anabolism-building
Homeostasis- balance what’s normal healthy checked when we get a physical.
Pathophysiology-when we get out of homeostasis.
Ischemia
Decreased blood flow to tissue. Any restriction in blood flow. For example, coronary artery disease. All tissues are subject to this.
Leads to hypoxic tissue but not all hypoxia is this.
Hypoxia
Tissue reduced oxygen level.
Commonality Ischemia and Hypoxia
Both result in decreased oxygenation in cell or tissue. Cell can’t produce ATP as well. As O2 decreases H+ gradient decreases ATP production decreases.
Lactate-lactic acid does not bulid up in cell. pH is too low. Converts to lactate and is converted back to pyruvate.
Cellular Stress
Toxins, metabolic problems (ex: diabetes), Neurodegenerative diseases (Parkinson’s, HD, Alzheimer’s), Ischemia/reperfusion, Inherited conditions (HD), physical trauma, and inflammation these lead to…
Proteopathy (alterations in protein), Metabolic stress, Mitochondrial stress, Oxidative stress (reactive oxygen species (ROS), Reactive Nitrogen Species (RNS), ER stress (ER hide Ca2+ when cell is at rest. Can’t leak Ca2+ bad things happen), and DNA damage (DNA itself doesn’t do a thing. Codes for proteins altered DNA leads to altered protein expression)
This leads to autophagy. Bad proteins made grab it and break it down. These leads to cell death if cell is to damaged or adaption.
Process of Autophagy
Lysosome- garbage collector of the cell
Take old organelles, altered proteins to their base component. ex: lipids, amino acids, etc…
Sequestration-autophagosome is formed around cytoplasm and organelles
Transport to a Lysosome- Autophagosome fuses with lysosome.
Degradation- Lysosome releases enzymes that degrade material in autophagosome
Utilization of degradation products- Recycle. All cellular materials degrade to amino acids. Anything that is old can be lipids too.
Cellular Stress Flow Chart
Atrophy
Decrease in cellular size. Can be positive or negative. Nutrient reduction is a good physiological adaption.
Musculocutaneous Impingement- nerve pinched lost resting muscle tone stimulus. Bicep not getting stimulated muscle shrank. Couldn’t do a 5 pound curl. Arm recovered after surgrey to remove impingement,
Hypertrophy
Increase in cell size. Can be bad in the heart. Inclusion blocks artery heart has to beat harder and faster. Lumen gets smaller decreases ejection fraction.
Hyperplasia
Increase in cellular number. Can be positive or negative. Compensatory- binge drinker scar tissue don’t drink for a few months. Liver will compensate and grow new tissue for what is lost.
Pathologic- too much growth. ex: keloid scar too many cells grow.
These cells go toward cancer, Not all these cells are cancer.
First step to cancer but not precancerous state though
Metaplasia
Swap cell types. Always Bad. Smokes cigarette causes repeated damage to trachea. Pseudostratified ciliated columnar epithelium usually line it. Smoking can cause stratified squamous to line it and it can’t do the mucocillary escalator so it causes them to have more lower respiratory infections. Quit smoking 10-12 months reverse adaptation. Go back to normal. Step toward Cancer.
Dysplasia
Next step past this is cancer. Precancerous state. Cell gives up normal shape and no longer do normal function, Most negative of the adaptations. Can reverse, but very unlikely. Muscle cells and neurons usually don’t do this and metaplasia locked in mature state.
Methods of cellular injury
Lack of Oxygen-hypoxia and ischemia. Decreased O2 decreases ATP production. Na+/K+ pump efficiency decreased loss of gradient decrease secondary active transport.
Free Radicals- Oxidative stress ROS/RNS lipid peroxidation. C=C essential to remain fluid. Lipid peroxidation causes C-C which makes the cell rigid and easy to rip cell.
Toxic Chemicals- lead, carbon tetrachloride. Lead binds to Ca2+ channels. 1st symptoms numbness in fingers and in toes for adults. It is much worse in children. Forming brain won’t make connections because blocked during neuroplasticity where connections should be made. Lead sits in the body for the long time. Carbon tetrachloride- dissolves organic material cleans clothes quick lots of dry cleaners died of kidney failure because it dissolves kidneys.
Infectious Agents- Bacteria and Viruses Mainly. Viruses can insert DNA. Bacteria and fungus generally cause damage by biproducts. Chronic Inflammation from bacterial virus can cause cell death.
Physical Trauma- Million ways to hurt yourself stub toe, shot, stabbed, etc…
Biochemical Mechanisms Cellular Injury
Adenosine triphosphate (ATP) depletion. No secondary active transport
Mitochondrial damage increased ROS
Accumulation of oxygen and oxygen derived free radicals- oxidative stress
Membrane damage (ATP depletion)- mitochondria or cell itself
Protein folding defects- DNA damage associated with infective agents ex: prion diseases, radiation
DNA damage defects
Calcium Level Alterations- abnormal influx or abnormal circulating Ca2+ can lead to biochemical alteration can also lead to calcification of things.
Cellular Injury can lead to death by: Oncosis
Decreased ATP production
Failure of active transport mechanisms (Na+/K+ pump)
Cellular swelling
Detachment of ribosomes from ER
Cessation of proteins synthesis
Mitochondrial swelling from calcium accumulation
Vacuolation. Return ATP is reversable.
Leakage of digestive from lysosomes: autodigestion of intracellular structure.
Lysis of plasma membrane
Death- necrosis ribosome pops off don’t make anymore of those proteins.
Necrosis and Oncosis Chart
Ischemic Stroke- blockage of blood flow, Immediate neuronal dysfunction. As this precedes oncosis continues swelling spread. Fix is to reopen the artery to try and reduce collateral damage.
Ischemic heart disease- heart muscle can die before heart attack.
Reperfusion Injury
Ischemic stroke- tight time window. Above 6 hours to be able to administer clot busting medication for positive effect. If wait more than 6 hours this occurs.
Free Radicals
ROS/RNS strongly oxidizing elements.
Superoxide is the most common one. These lead to lipid peroxidation. Same with protein. Changes shape. Changes DNA shape. Can’t describe DNA.
Free Radical Injury
Oxidative stress number one theory we age. 3-5% of electrons leak out of mitochondria create free radicals. Superoxide dismutase detoxifies superoxide. Antioxidant enzymes extremely important 10% ALS caused by defective SOD mutation. Shows up early in life. Antioxidant vitamins mixed results. Excessive vitamin C may produce free radicals also water soluble don’t use it. Vitamin E protects lipid peroxidation. Vitamin E regenerate by Vitamin C not 100% helpful.
Chemical Injury
Direct toxicity to cell. Damage to or destruction of plasma membrane. Reactive free radicals and lipid peroxidation.
Lead blocks Ca2+ channels. Carbon monoxide binds 300x tighter to hemoglobin than O2. It is irreversible leads to hypoxia.
Ethyl alcohol- acetaldehyde binds to phospholipids, amino acid residues. Leads to failure of DNA repair, mitochondrial abnormalities, impairment of microtubular function, and failure of the cell membrane. Acetaldehyde extremely toxic the more someone drinks the more this bulid’s up gives hangover gives rigid spots leads to cell lysis.
Oncosis, Necrosis and Apoptosis
Oncosis- reversable. swelling of endoplasmic reticulum and mitochondria. Membrane blebs.
Necrosis- breakdown of plasma membrane organelles and nucleus leakage of contents. Cell basically falls apart.
Apoptosis- macrophage eats it. Occurs in development of fingers sculpted from this. Normal cell, enzyme activation causes the cell to shrink and chromatin to condense. membrane starts blebbing organelles disintegrate, nucleus and organelle membrane continue, apoptotic bodies form, macrophages phagocytose apoptotic bodies, no inflammation.
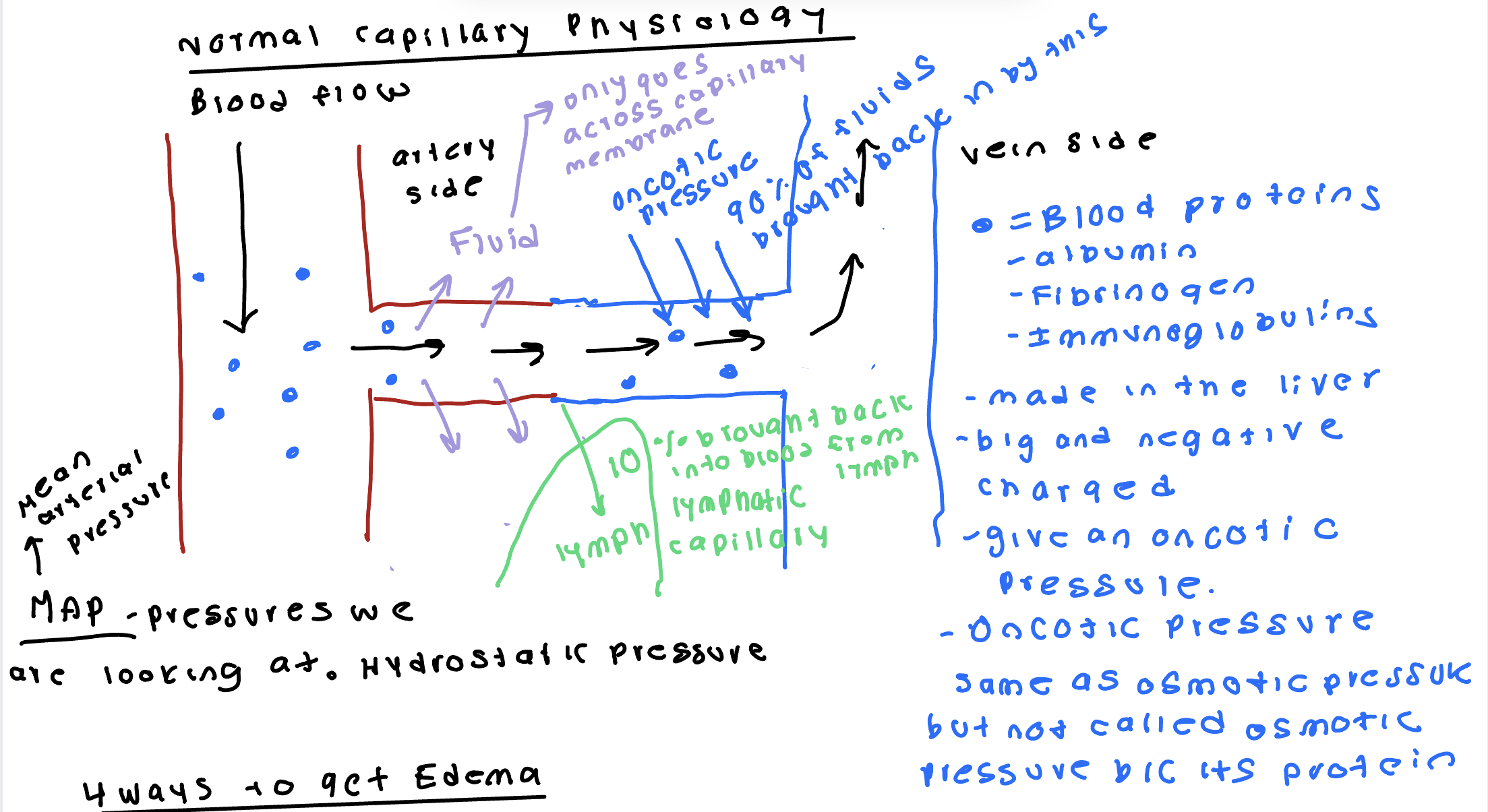
Normal Body Water Distribution and Edema
2/3 intracellular fluid 1/3 extracellular fluid is normal. ¼ plasma and ¾ interstitial fluid.
Edema- excessive interstitial fluid. Come side effects of diseases and disorders. Easy to see.
Ways to get Edema
In mean arterial pressure- hypertension can lead to systemic edema.
Liver disease/ malnutrition- decreases oncotic pressure. Malnutrition leads to less protein being made. Ex: people in famine eat no protein. Can’t make protein without eating protein. Causes oncotic pressure decreases causing fluid to be left behind.
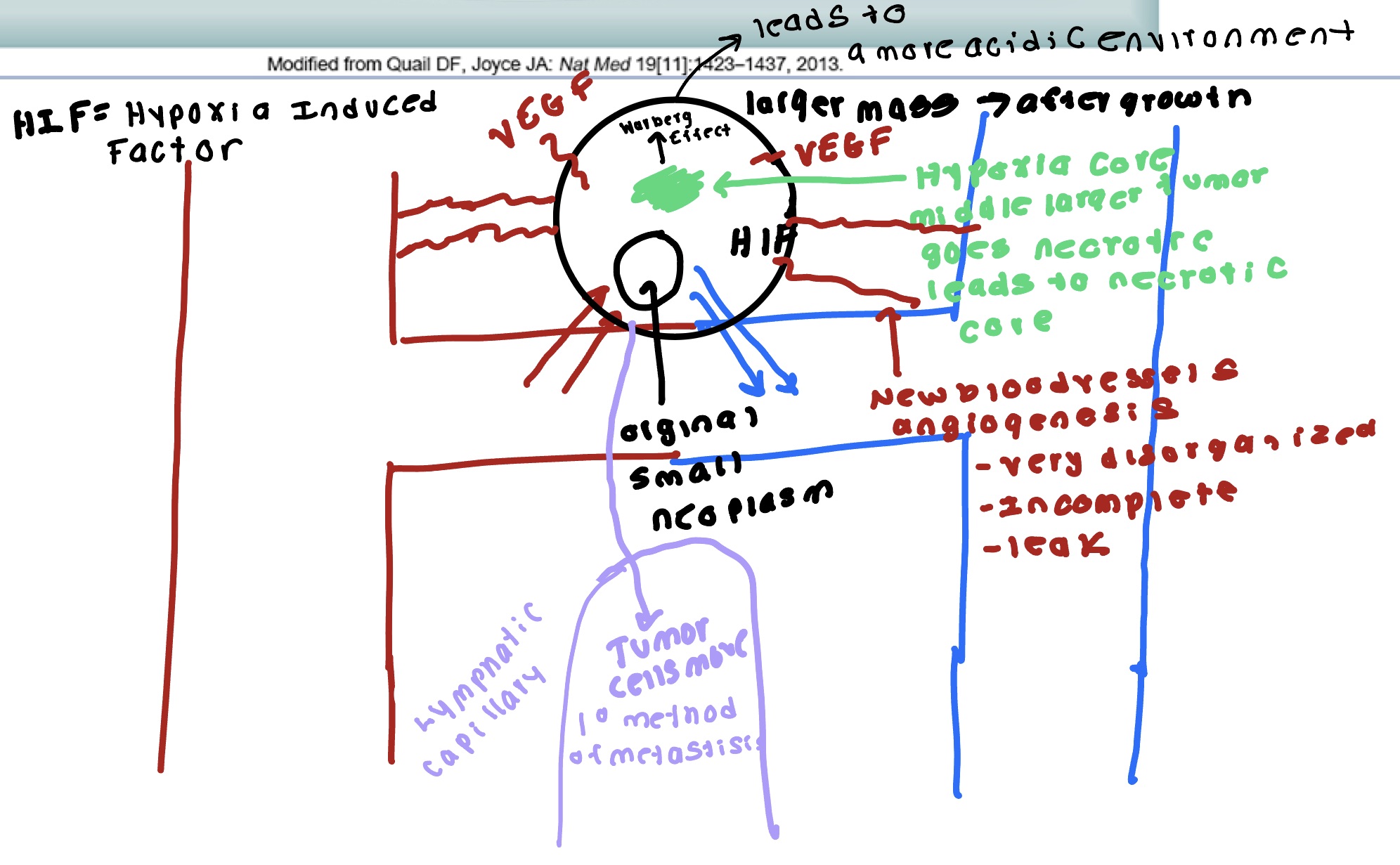
Blockage of removal of lymphatic capillary- removal occurs as a cancer treatment. Left axillary lymph nod removed can’t take blood pressure off left arm. Leads to swelling 3-5 days. Blockage can occur with metastasis and bacterial infection possible sepsis.
Inflammation- releases histamine. Histamine increases permeability of capillary membrane. More fluid goes in and only the same amount comes out. Acute inflammation not a big deal. Chronic inflammation can cause tissue dysfunction.
Reasons are additive on each other and make things worse.
Hypernatremia
Normal levels 136-145 mM
Hypovolemic- greater decrease in H2O and same decrease in Na+ concentration. Loop diuretics cause this. Ex: Lasix is an example. Osmotic diuresis diabetic patients, excessive glucose in blood will pull fluid. GI losses- diarrhea and vomiting. Kidney Failure.
Isovolumic- loss of pure H2O that results in an increased Na+ concentration. Not drinking enough, excessive sweating, fever with hyperventilation, burns 3rd degree, diabetes insipidus decrease in ADH.
Hypervolemic- rare. Too much salt and a little extra water. most often caused by IV administration of Na+. Increase aldosterone. Increase ATCH comes from tumors in the adrenal gland.
All lead to intracellular dehydration
Hyponatremia
Hypovolemic- little decrease in H2O and large decrease in Na+. Vomiting, diuretics, decreased aldosterone.
Isovolumic- decrease in Na+ SIADH, dilutional hyponatremia- drink to much pure water. Can be seen on psych ward patients with meds drink lots of water.
Hypervolemic- increase in H20 potentially slight increase in Na+. Heart failure, cirrhosis, and renal failure all lead to H2O retention.
In all of these, cell swelling is seen. Chronic alcoholics- remove alcohols. They will put themselves in dilutional this.
Hyperkalemia
Increase in K+
Increased K+ intake- common in patients with sodium restriction using KCl salt. Can’t overdose on bananas.
Hypoxia- shift of intracellular K+ extracellular. Na+/K+ ATPase. As ATP levels drop can’t reestablish gradient. K+ stuck on the outside. K+ channels keep leaking can’t be pumped back.
Acidosis- increased H+. Results in this.
Get muscle weakness from result on resting membrane potential. Weakness due to higher resting membrane potential in cells. Increase rate of repolarization leads to tachycardia.
Hypokalemia
Reduced intake of K+
Renal losses of kidney failure
Respiratory alkalosis
Too much insulin stimulates K+ uptake
More negative harder to depolarize leads to muscle weakness. Leads to slow heart rate= bradycardic slow because hard to repolarize heart to beat again.
Hypercalcemia
High concentration of Ca2+ in interstitial space. Leads to bone weakness, kidney stones, GI distress, inhibit depolarization when severe (muscles and neurons) and impaired mental states. Causes shortened QT interval.
Causes of this:
Malignancy- when it travels through lymphatics to distant site. Sets up second tumor in distant site. Primary tumor breaks through basement membrane metastasis then spreads to become maligency.
milk-alkali syndrome
Vitamin D toxicity- Vitamin D needed for Ca2+ absorption. Too much of this potential for this.
Paget’s disease
Hyperparathyroidism- PTH increases retention of Ca2+ in kidneys, increased intestinal absorption, increased osteoclast activity. Usually side effect of transformation.
Immobilization- long term this leads to this. Osteoclasts outpace osteoblasts from not using it.
Granulomatous disease
Thiazide diuretics.
Acidosis- denaturation of transport proteins release of Ca2+ in fluid.
Hypocalcemia
Common causes: hypoparathyroidism (surgical removal or damage, thyroid disease or autoimmune), vitamin D deficiency and insufficiency (decreased Ca2+ absorption- chronic kidney disease), altered vitamin D metabolism due to medication usage, disease affecting the kidneys and or the liver, pseudohypoparathyroidism, hypomagnesemia or hypermagnesemia, hungry bone syndrome, infusion of phosphate, rapid citrated blood transfusion, and medications.
Effects: increased neuroexcitability inappropriate muscle contraction. leads to prolonged QT interval- long repolarization leads to arrhythmic heart rate.
Hypophosphatemia
Neuromuscular- muscle, weakness, tremors, paranesthesia, bone pain, hyporeflexia, seizures, delirium, hallucination, ascending motor paralysis.
Hematologic- tissue, hypoxia, possible bleeding, possible infection.
Cardiopulmonary- weak pulse, hyperventilation, respiratory weakness.
GI-anorexia dysphagia.
Hyperphosphatemia
Neuromuscular- tetany (with decreased Ca2+), hyperreflexia, muscular weakness (more common with hypophosphatemia), flaccid paralysis.
Cardiopulmonary- tachycardia
GI- nausea, diarrhea, abdominal cramps.
Hypomagnesemia
Neuromuscular- hyperirritability, tetany like symptoms, tremors, twitching of the face, spasticity, increased tendon reflexes.
Cardiac- Hypertension cardiac dysrhythmias- PVC’s, VT (torsades), VF, and flat or inverted T wave, ST depression (like low K levels).
Hypermagnesemia
Neuromuscular- CNS depression, lethargy, drowsiness, weakness, paralysis, loss of deep tendon reflexes.
Hypotension, complete heart block (3rd degree), bradycardia, widened QRS complex, prolonged QT interval.
Others: flushing, respiratory depression.
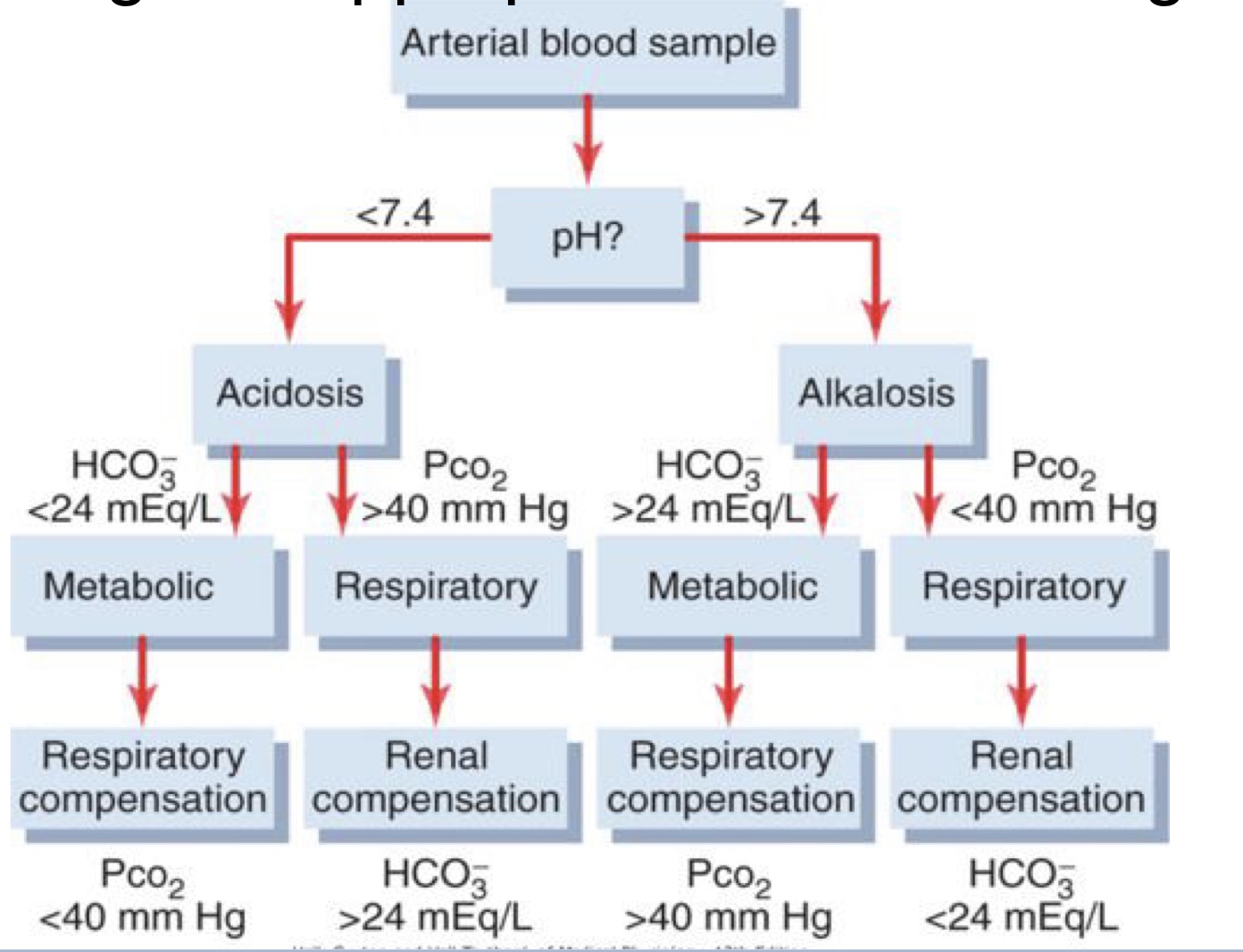
Describe the role of pH, Pco2, and bicarbonate in evaluating acid-base imbalances
Action of buffer to stabilize the pH. Bicarbonate buffer is the dominant buffer. Normal pH 7.35-7.45
CO2 and Water make carbonic acid which make H+ and bicarbonate ion. All these reactions are reversable. carbonic anhydrase help the transfer from CO2 and water to carbonic acid. CO2 get rid of by lungs. H+ excreted by kidney HCO3 kidney reabsorbs. Use Le’ Chatlies principle to help maintain normal balance.
Metabolic Acidosis lecture
Increased acid production decrease in O2 availability (hypoxia)
Increase loss of bicarbonate- diarrhea and renal tubular acidosis.
Acid secretion- renal failure and chronic kidney disease
Rare increase in acid consumption.
Decrease pCO2 leads to hyperventilate.
Metabolic Alkalosis
Hypokalemia H+ shifts into cells
GI loss vomit
Antacid overdose
Hyperaldosteronism- increase aldosterone greater renal loss of protons.
Secondary response increase pCO2 hypoventilation
Respiratory Acidosis
Decreased pH and increased H+. Increased pCO2 and increased in bicarbonate renal compensation.
Respiratory alkalosis
Increased pH decreased H+. Decreased pCO2. Response is a decrease in bicarbonate.
Acidosis and alkalosis chart
Benign
Small, well demarcated, slow growing, Non-invasive, nonmetastatic, and well differentiated. Can tell us what tissue it is.
Not cancer because it doesn’t have the ability to spread. Ex: polyps in the colon. Can be pre-cancerous, but not cancer.
Encapsulated, localized and limited in size.
Malignant
Large, poorly demarcated, rapidly growing with hemorrhage and necrosis. Tissues are growing so fast producing blood vessels to feed tumor. Core of tumor dies because it is to far from the blood supply. Locally invasive, metastatic poorly differentiated. Step backward and sideways. No longer looks like an adult cell or does its function. Doesn’t visibly look like the cell it came from. Cancer will spread. Rarely hear of muscle or nervous cancer because can’t go back into mitosis. If cancer in brain, it is in the glial cells.
Can continue to grow by breaking through basal laminae and invading adjacent tissue.
One mutation won’t cause cancer multiple will. Not exact line because cancer is individualized One oncologist said at least 15 different mutations.
Neoplasm
New growth of non-normal tissue benign or malignant dedifferentiated tumor. Has not broken the basement membrane.
Dysplasia- pre-cancerous loss of normal cellular shape and function not over growing yet. Not rapidly dividing. In this they are rapidly growing and dividing.
Characteristics of cancer cells
Transformation- dedifferentiation- doesn’t look/do what it supposed to do change in appearance/ lack of normal function.
Anchorage-Independence- Normal cells must be attached to basement membrane. Cancer cells lose anchorage dependents.
Immortal- They don’t die. In transformation turn off cell clock. As long as you feed it they will keep dividing and growing. Don’t care about aging process.
Anaplasia- Loss of any kind of structure. Loss of mature cellular features.
Pleomorphic- ability to produce a lot of different stuff that they normally shouldn’t be able to produce ex: ADH produced by liver tumor. Variable size, shape, and color.
Clonal Proliferation- Can mutate on top of themselves more aggressive transformation. Can get more mutations can get worse.
Tumor Cell Markers
Made by cancer cells or made in response to cancer cells
Prostate Specific Antigen (PSA)- PSA protein made at a very low level in healthy young males. As aging occurs it goes up. Most part PSA abandoned as for being cancer. Abnormal number is considered to be 2.5
Tumor Cell Markers Used
Screening if family history.
Diagnostic
Staging Cancer/ determining aggressiveness
Is treatment working
Promotion of Tumor Growth/ cellular transformation
Autocrine stimulation- self stimulation, Through release of growth factors then respond to it themselves.
Increase in growth factor receptors- increase in sensitivity.
Mutated cell-surface receptors- always activated doesn’t need a ligand anymore
Mutation in tumor promoter- oncogenes
Inactivation of tumor suppressor
Oncogene
Protooncogene- does normal job mutated to form this. Same gene but no longer regulated. Leads to uncontrolled cellular division.
Tumor Suppressors
Block the cell cycle. Can hold cell in G1 or from going to G0 (adult cell doing its job and function). Mutation takes away break that keeps cell division from occurring. Allows for uncontrolled cell growth.
Tumor promoter and cell cycle progression
RAS pathway common mutation types in many types of cancers. These enzymatic pathways promote production of the myc protein. MYC proteins are transcription factors that activate expression of pro-proliferative genes.
P53
Tumor suppressor. Involved in many cancers because many significant functions. Mutant cells expand in number then have genomic instability. Easier to have more mutations at faster rate than normally would.
Telomerase in tumor development
Telomeres- what is left over after replication. Mutation of this leads cells to become immortal.
Removal of the RNA primer leads to the shortening of the chromosome after each round of replication. Chromosome shortening eventually leads to cell death. As RNA sequence in this acts as a template for DNA. This enzyme adds the telomeric sequence to the 3’ end of the chromosomes. The original length of the chromosomal DNA has been restored. Note the gap where the primer for DNA replication has been removed.
Accumulation of Mutations
multistage theory of carcinogenesis
First mutations- inactivation of APC. Cell seems normal but is predisposed to proliferate excessively.
Second mutation- mutational activation of K-ras. Cell begins to proliferate too much but is otherwise normal.
Third Mutation- Loss of DCC, over-expression of COX-2. Cell proliferates more rapidly. It also undergoes structural changes.
Fourth or later mutations- Loss of TP53 activation of telomerase. Cell grows uncontrollably and looks obviously abnormal.
Oncogenic Activation
Point mutations- either base is missing or incorrect base is used.
chromosome translocations- more on chromosomes form new genes.
Gene amplification
Tumor suppressor gene mutation
Loss of heterozygosity
Translocation of Chromosome Ends
ABL and BCR translocate ends. Makes ABL-BCR oncogene only on one chromosome. This leads to tyrosine kinase which is growth promoting.
Warburg Effect
All transformed cells have this altered metabolism. Inefficient use of glucose that all cancers use. Cancer cells choose to do anerobic pathway. Makes 4 mol ATP/ mol glucose.
Chronic Inflammation in cancer development
Chronic inflammation- keep injuring something cell with transform in some way. Pathologic this can lead us towards a cancerous transformation.
Mutagens
Physical- Radiation. UV light ionizing radiation. Dimerization to thymine dimmer when UV light hits gene if tumor suppressor is turned off leads to cancer. Heat- can cook DNA. Saunas may not be good for you.
Chemical- Base analogs, Intercalating agents (insert themselves in DNA), alkylating agents, and Deaminating agents (add or remove things to DNA structure), and metals (can alter ability to read and express genes)
Biological- Transposon, Virus ( HPV vaccine. HPV #1 cause of cervical cancer), and bacteria
Germ-line mutation lecture
Heritable, maybe result from 1 mutation. Both sad and shocked rare occurrence. Entire organism carries the mutation. Half the gametes carry mutation. 70 mutations early onset or childhood cancer.
Somatic Mutations
342 mutations. Not passed onto offspring. Grey area some people born more susceptible for cancers. Somatic mutation, patch of affected area, and none of the gametes carry mutation.
Viral Oncogene
HPV inserts into genome it degrades p53 and inactivates RB tumor suppressor
This expression and viral and cellular oncogene expression leads to proliferation, survival, DNA repair mechanism, genomic instability, telomeres length/ senescence, and cell polarity.
Carcinoma development and acquisition of invasive potential
Tumor metastatic genes, metastatic suppressor genes.
Expansive growth and invasion of basement membrane into surrounding tissues- enhanced protease activity (ex: MMPs- dissolve ECM), Enhanced cell motility/ interaction with surrounding tissue/ECM/stromal cells, Decrease integrity strength of cell-cell contacts (E-cadherin TJ’s etc.. Acidic environment)
Angiogenesis, intravasation and transport around body- migration/ interaction through ECM, interaction with vascular cells, interaction with cavity mesothelial cells ( during Transcoelomic metastasis), invasion into blood vessel, and survival in circulation/ immune evasion.
Arrest and extravasation at secondary site- interaction wit vascular cells, interaction with mesothelial cells during transcoelomic metastasis, invasion into secondary tissue with help of neutrophils.
Invasion of secondary tissue and formation of micro- or macro metastasis- invasion/migration into secondary tissue, interaction and adaption to tissue microenvironment, establishment of new vasculature, secondary tumor establishment or dormancy.
Cancer Spreading
Epithelial- mesenchymal- growing tissue, replicating tissue. EMT (epithelial mesenchymal transition) acidic environment stimulates this.
EMT
Results: loss of cell adhesion, loss of polarity, gain migratory and invasive characteristics. Start to crawl and can now move wherever they want to go, Acid environment breaks down extracellular matrix.
This leads to an invasion to adjacent tissue and circulation. → TGF-B - stimulates fibroblasts to make way for growing cells degrade ECM. CSF-1- makes tumor associated macrophages (TAM) ability to crawl around move degrade things, supports further metastasis. → recruit platelets. Platelet coat protects from sheer forces, protect from immune and inflammatory cell destruction, platelets recruit neutrophilic action to degrade ECM at site of invasion→ invasion.
Location of Secondary Tumors
Primary- lung and bone
Secondary- Liver, brain and cerebrospinal fluid
Tertiary- Adrenals
All of these spots have a high degree of vascularity.
Angiogenesis
Tumor secretes VEGF- HIF
VEGF increases blood vessel expression and movement of tumor.
Tumor has increased blood supply- leaky vessels not well formed.
PDGF- stimulate growth of blood vessels
bFGF- assist in building blood vessels.
Inhibition of Angiogenesis
Monoclonal antibodies that specifically recognize and bind to VEGF. When VEGF is attached to these drugs, it is unable to activate VEGF.
Bind to VEGG and/ or its receptor as well as to other receptors on the surface of endothelial cells or to other proteins in the downstream signaling pathways, blocking their activity. Great if keep monoclonal antibodies local. Can’t be on these meds forever. Precent healing from occurring.
Staging Cancer tumor node metastasis
T= primary tumor; the number equals size of tumor and its local extend. The number can vary according to the site. T0= breast free of tumor, T1= lesion <2cm in size, T2=lesion 2-5 cm. T3= skin or chest wall involved in the invasion.
N= lymph node involvement, a higher number means more nodes are involved. N0- no axillary nodes involved, N1 mobile nodes involved, N2-fized nodes involved.
M= extend of distant metastases. M0- no metastases, M1-demostratable metastases, M2- suspected metastases.
Group Staging Cancer
0. Abnormal Cells are present but have not spread to nearby tissue
I. Early stage: cancer has spread to other tissue in small area.
II. Localized- tumor is between 20-50 mm and some lymph nodes are involved or a tumor larger than 50 mm with no lymph nodes involved.
III. Regional spread- tumor is larger than 50 mm with more lymph nodes involved across a wider region. In some cases, there is no tumor present at all. Cancer may have spread to skin or chest wall.
IV. Distant Spread- cancer has spread beyond the breast to other parts of the body.
Common Clinical Manifestations of Cancer
Anemia- If red marrow gets invaded and mass gets in there. Normal cells contact inhibition- when pressure gets put in there induces apoptosis- so normal cells go through apoptosis and RBC’s can’t be made as well.
Leukopenia- reduce production of WBC’s
thrombocytopenia- not producing enough platelets.
Infection- opportunistic injections comes from leukopenia.
Paraneoplastic syndromes- inappropriate production of cellular product which make something it shouldn’t
Fatigue and pain- most common symptoms.
Cancer patients hard time eating, nauseous no appetite. As mass breaks out of tissue puts pressure on other tissues.
Cachexia
Involuntary weight loss- occurs despite getting adequate nutrition or high number of calories.
Muscle wasting- this is a characteristic symptom.
Loss of appetite or anorexia- not only does food become not appealing, but a person with this will also lose their desire to eat any food.
Reduced Functional Ability- common symptoms such as malaise, fatigue, and low energy levels make it hard for a person to do the things they enjoy and want to do.
Swelling or Edema- when there are low levels of protein in the blood, fluid moves into the tissues causing swelling. Due to malnutrition decreased protein levels blood leads to decreased oncotic pressure.
Chemotherapy
Targets mitotic (dividing cells) slows/ shrinks the cancers to decrease tumor load. GI side effects, hematopoietic side effects. Has to be given in doses because it is toxic. Procrit increases hematocrit. Leaves you feeling poorly and immunocompromised.
Surgery
Try to debulk the tumor. Difficult because malignant tumors have feels and appendages and have spread all over. Can do PET scan with radioactive glucose to show surgeon where the tumor is. An extreme case is surgery mixed with chemotherapy. Person who gets surgery and the wash with high temp chemotherapy. Then was chemotherapy out. 40% survival rate. Very hard on patient.
Radiation
Using a cancer causing event to kill cancer. Highly effective but have disorganized mass only kill part of the cancer, so multiple treatments followed by chemotherapy at the same time. Now do surgery followed by this and chemotherapy.
Brachytherapy- radioactive beads for bladder cancer. Sits in the bladder.
Immunotherapy
Very effective in some of the leukemias. Large intestine mass hard to find one type of antigen because of rapid mutations. Very heterogenous. Difficult to get an immune target. Very individualistic therapy so very time consuming and costly.
Anabolism
The energy using process of metabolism
Catabolism
The energy releasing portion of metabolism.
Role of ATP
Universal fuel in living cells. The fuel drives biologic reactions necessary for cell function. When 1 mol of glucose metabolically breaks down in the presence of oxygen and CO2 and water 686 kcal of chemical energy are released. The chemical energy lost by one molecule is transferred to another molecule by an energy carrying or energy transferring molecule such as this molecule.
The energy stored in this can be used in a variety of energy requiring reactions and in the process is generally converted to ADP and Pi. This is used for muscle contraction, and active transport.
Process Breaking down Food for Energy
Digestion- Larger molecules are broken down into smaller subunits: proteins into amino acids, polysaccharides into simple sugars, and fats into fatty acids and glycerol. These processes occur outside the cell within the intestines or within the specialized lysosome organelle within the cell and are activated by secreted enzymes.
Glycolysis and oxidation. The most important part of phase two of glycolysis, the splitting of one glucose molecule into two molecules of pyruvate. During pyruvate formation, ATP and reduced NADH are produced through oxidation, or the removal and transfer of a pair of electrons. The total process is called oxidative cellular metabolism involves 10 biochemical reactions.
Citric acid cycle- most of the ATP is generated during this final phase, which begins with the citric acid cycle and ends with oxidative phosphorylation. Approximately 2/3 of the total oxidation of carbon compounds in most cells is accomplished during this phase. The major end products are CO2 and NADH and FADH2 both of which transfer their electrons into the electron transport chain.
Oxidative phosphorylation
Occurs in the mitochondria and is the mechanism by which the energy produced from carbohydrates, fats, and proteins is transferred to ATP. During catabolism of foods many reactions involve the removal of electrons from various intermediates. These reactions generally require a coenzyme such as NAD to transfer the electrons and thus are called transfer reactions.
Molecules of NAD and FAD transfer electrons they have gained from the oxidation of substrates to molecular O2. The electrons from reduced NAD and FAD, NADH and FADH2 respectively, are transferred to the ETC on the inner surfaces of the mitochondria with the release of hydrogen ions. Some carrier molecules are cytochromes which accept electron pairs. These electrons eventually combine with O2.
Anaerobic Glycolysis
WIthout O2. Linked to the breakdown of carbohydrates. Because this occurs in the cytoplasm of the cell, it provides energy for cells that lack mitochondria. The reactions involved in the conversion of glucose to pyruvic acid with the simultaneous production of ATP. 1 molecule glucose produces two molecules of ATP and pyruvate. If O2 is present, the two molecules of pyruvate move into the mitochondria where they enter the citric acid cycle.
If O2 is absent pyruvate is converted to lactic acid which is released into the ECF. Pyruvic acid to lactic acid is reversable, therefore once O2 is restored lactic acid is quickly converted back to pyruvic acid or glucose.
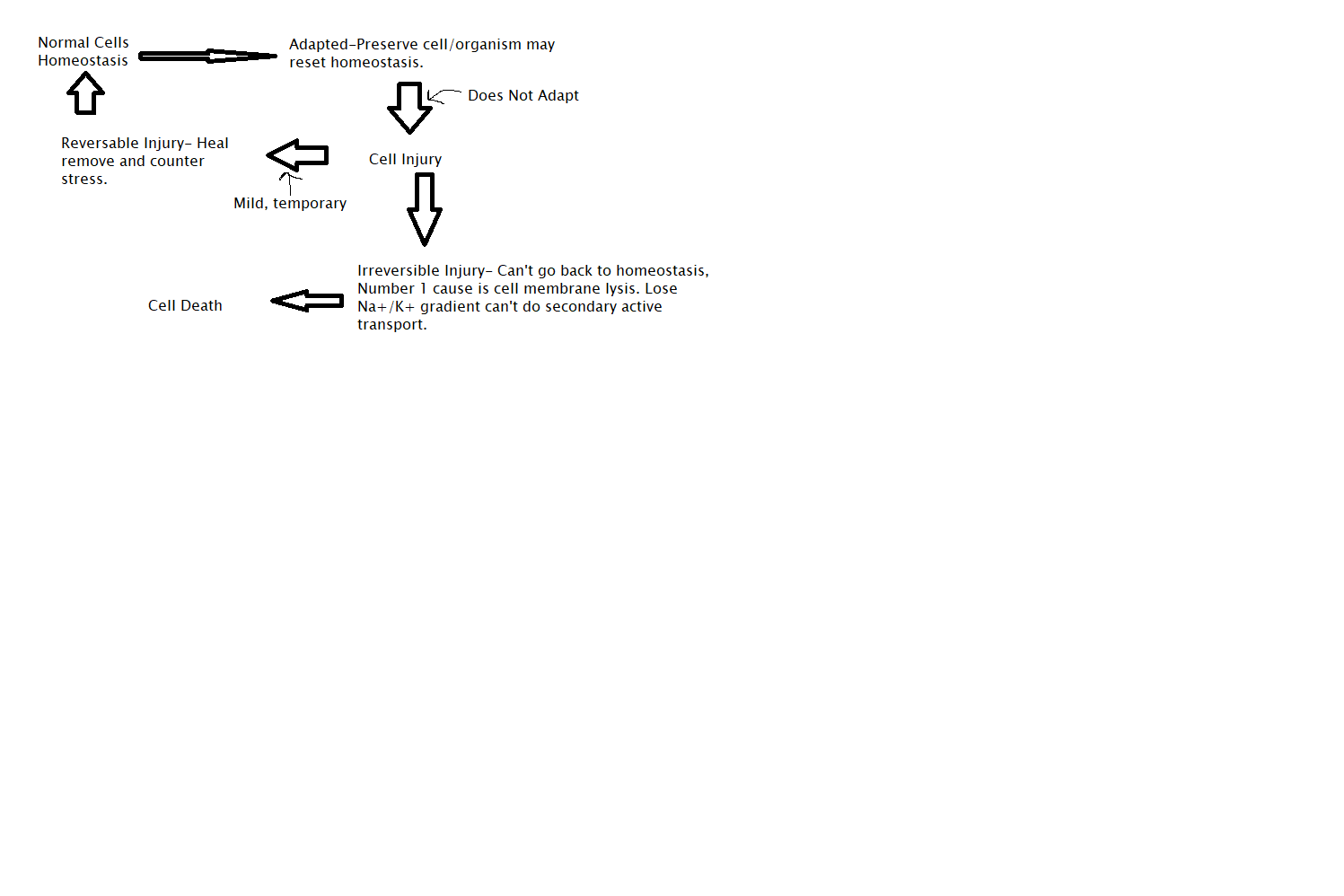
Cellular Adaption
Adapted cell is neither normal or injured it falls somewhere between those two states. These are reversable changes affecting the size, number, phenotype, metabolic activity or function of those cells. These have limits additional stress can compromise essential cell functions leading to cell injury or death. In the early stages of this, cells have enhanced function, making it difficult to distinguish a pathologic response from vigorous adaption.
Atrophy
Decrease in cell size. If this affects enough cells, the affected organ decreases in size. It can affect any organ but it occurs most commonly in skeletal muscle, heart muscle, secondary sex organs, and the brain. Physiologic- occurs with normal development. Ex: tonsils shrink, and thymus gland decrease.
Pathologic occurs in organs as a result in decreases in workload, pressure, use, blood supply, nutrition, hormonal stimulation, or neural stimulation. Disuse- occurs when a limb is placed in a cast for an extended period with bed rest or immobilization.
Hypertrophy
Is a compensatory increase in size of cells occurring in response to mechanical loads or stress and results in increased size of the affected organ. Common triggers include repetitive stretching, chronic pressure, and volume overload. The cells of heart and kidney are especially prone to this. This is an adaptive response.
Physiologic- results from increased demand, stimulation by hormones, and growth factors.. Sn example is runners heart
Pathologic- results from hemodynamic overload such as from hypertension or heart valve dysfunction.
Hyperplasia book
Is an increase in the number of cells resulting from an increased rate of cellular division. As a response to a stimulus, this occurs when the damage is severe or prolonged or when it results in cell death. Requires that cells undergo mitosis a process wherein a single cell divides into two identical cells. Main mechanism for this is the production of hormones or growth factors which stimulate the remaining cells after injury or cell loss to synthesize new cell components and ultimately divide. Another mechanism is increased output of new cells from tissue stem cells.
Compensatory this is an adaptive mechanism that enables organs to regenerate. Liver regrowth.
Hormonal- occurs chiefly in estrogen dependent organs such as the uterus and the breast.
Pathologic-abnormal proliferation of normal cells usually in response to excessive hormonal stimulation or to the action of growth factors on target cells
Dysplasia
Refers to abnormal changes in size, shape and organization of mature cells. Is not considered a true adaptive process but is related to hyperplasia. Common in epithelial tissue and uterine cervix, the endometrium, and GI and respiratory tract mucosa. This that does not involve the entire thickness of epithelium may be completely reversable. When it penetrates the basement membrane it is considered an invasive neoplasm.
Metaplasia
Is reversable replacement of one mature cell type by another cell type that can survive an adverse environment. It is found in association with tissue damage, repair, and regeneration. usually the change is not beneficial. Ex: in long term cigarette smoker ciliated columnar epithelial cells of the trachea and bronchi become replaced with stratified squamous epithelium. Do not secrete mucous or have cilia causing a loss of a critical protective mechanism.
Cellular Injury
Injury to cells and to the ECM leads to injury of tissues and organs and ultimately determines the structural patterns of disease. This occurs when the cell is unable to maintain homeostasis. May be reversable or irreversible. Loss of function is the result of the cell and ECM injury and cell death.
Mechanisms Cell Injury Book
ATP depletion- loss of mitochondrial ATP and decreased ATP synthesis results include cellular swelling, decreased protein synthesis, decreased membrane transport, and lipogenesis, all changes that contribute to loss of integrity of plasma membrane.
Reactive oxygen species- lack of O2 is key in progression of cell injury in ischemia (reduced blood supply) activated oxygen species cause destruction of cell membrane and cell structure.
Ca2+ entry- Normally intracellular cytosolic Ca2+ concentrations are very low, ischemia and certain chemicals cause an increase in cytosolic Ca2+ concentrations, sustained levels of Ca2+ continue to increase with damage to plasma membrane, Ca2+ causes intracellular damage by activating a number of enzymes.
Mitochondrial damage- Can be damaged by increased cytosolic calcium and ROS. Two outcomes loss of membrane potential which cause ATP depletions leads to death or necrosis of cell and activation of apoptosis.
Membrane damage- early loss of selective membrane permeability found in all forms of cell injury, lysosomal membrane damage with release of enzymes causing cellular digestion.
Protein misfolding DNA damage- proteins may misfold triggering unfolded protein response that activates corrective responses overwhelmed response activates cell succeed program or apoptosis DNA damage also can activate apoptosis.
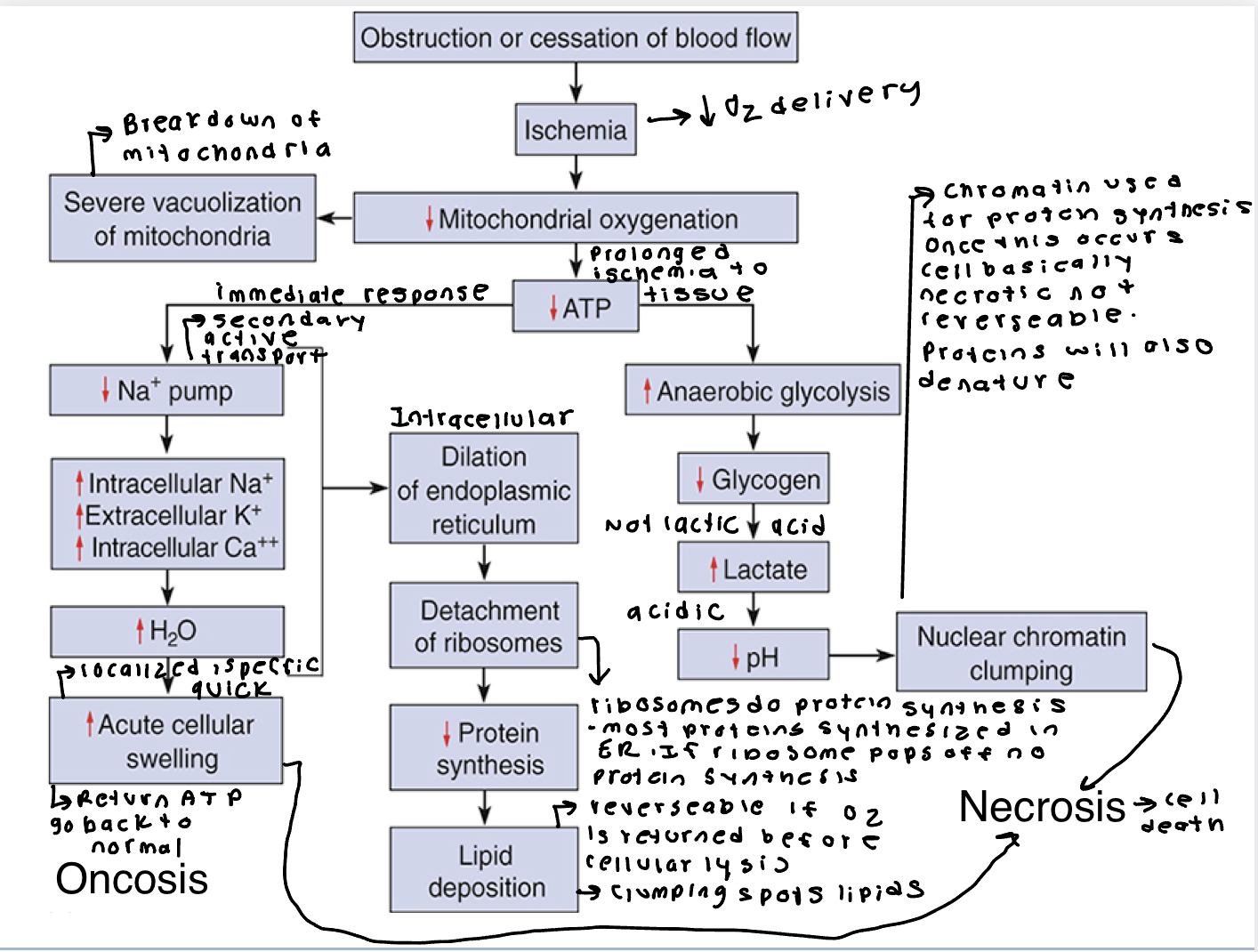
Ischemic or Hypoxic Injury
Single most common cause of cellular injury and is prominent feature of pathological states encountered in bacterial infection, inflammation, wounds, cardiovascular defects, and cancer.
Hypoxia- can result from several circumstances such as reduced oxygen content in the ambient air, loss of hemoglobin, decreased RBC production, respiratory and cardiovascular diseases, and poisoning of cellular oxidative enzymes.
Hypoxia triggers the mitochondrial complex to produced ROS, which promotes oxidative stress and can damage cell.
Ischemia induced reduction of ATP levels causes a failure of active transport mechanism. Without this mechanism sodium and water can freely enter the cell resulting in cellular swelling and dilation of the ER reducing protein synthesis. If it persists entire cell swells. Reversable is oxygen is restored. If not restored vacuolization occurs in the cytoplasm. Cell death rapidly follows as calcium accumulates in the cell, and metabolic processes cease.
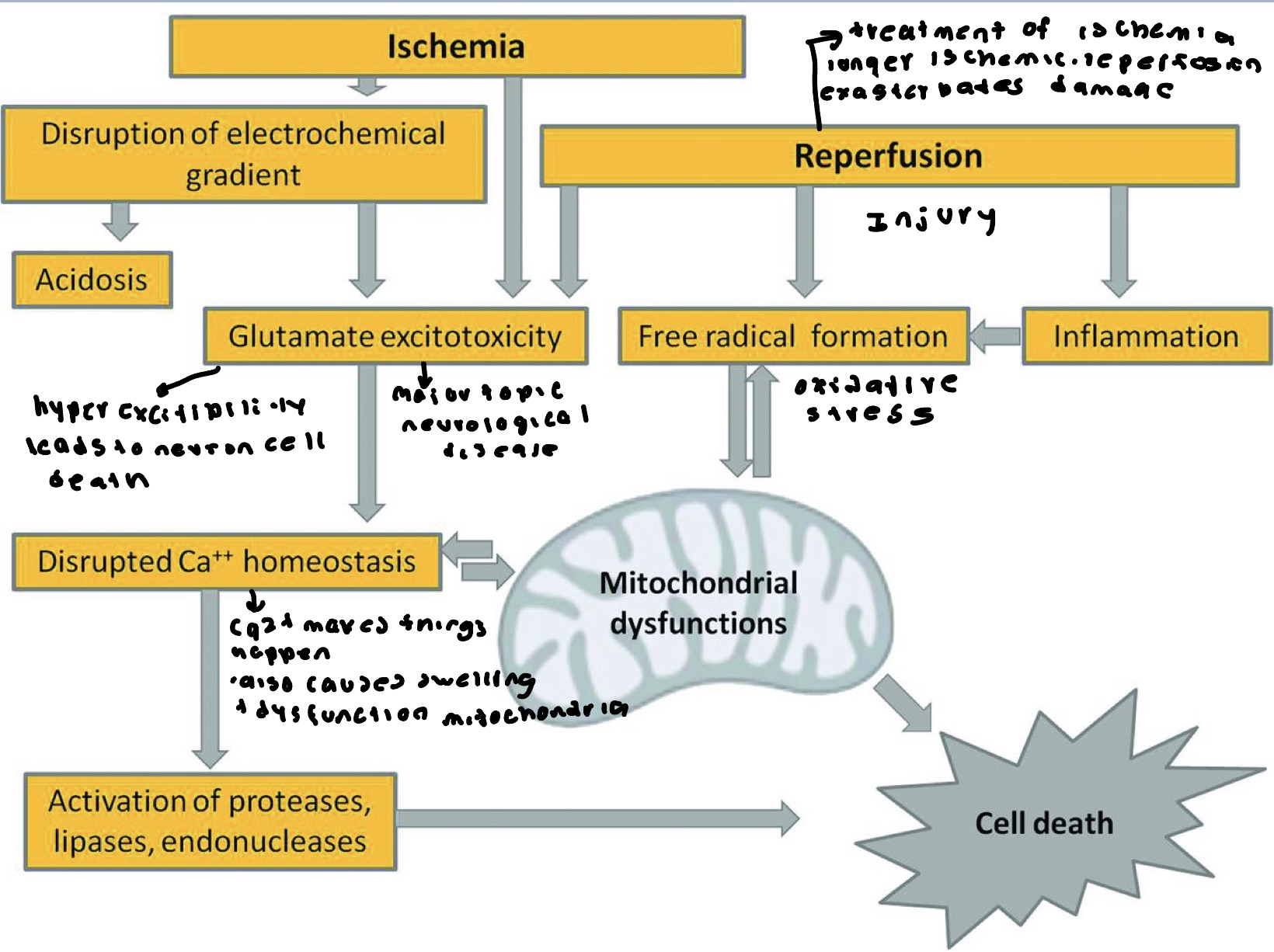
Reperfusion Injury
Serious complication and an important mechanism of injury in instances of tissue transplantation and other ischemic syndrome.
Oxidative stress- reoxygenation induces oxidative stress by generating ROS and nitrogen species. OH, O2-, ONOO-, H2O2- all have been shown to increase within minutes of reperfusion. They cause damage to the myocytes by altering membrane proteins and phospholipids.
Nitrogen based free radicals present mostly in the form of NO and are generated by endothelial cells macrophages, neurons and other cells. The radicals further damage the already compromised membrane and facilitate calcium overload within the mitochondria. This promotes proinflammatory neutrophil adhesion to the endothelium where they release toxic oxidants and harmful proteases. Antioxidant agents reverse this. They also reverse neutrophil mediated reperfusion injury in cardiac muscle.
Increased intercellular Ca2+ concentration- intercellular and mitochondrial ca2+ accumulate within the cell during acute ischemia. Reperfusion results in even more Ca2+ influx because damaged cell membrane and ROS mediated injury to the SR. The increased calcium enhances mitochondrial permeability and decreases and ceases ATP production.
Inflammation- Dead cells stimulate immune cells to release cytokine mediated danger signals thus initiating an inflammatory response
Complement activation- may exacerbate microvascular damage that occurred secondary to this.
Oxidative stress
is caused by an increase in different reactive species, depletion of antioxidant defense or both. Results in detrimental oxidation of different molecules
How are free radicals formed
Redox reactions
Absorption of extreme energy sources
Enzymatic metabolism of exogenous chemicals or drugs
Transition metals
NO
Examples free radicals
ROS- generated either directly during autoxidation in mitochondria or enzymatically by enzymes in cytoplasm. Can be deactivated by SOD or spontaneously.
H202- catalase can break this into O2 and Water
OH-
NO
Free Radical Effects On cells
lipid peroxidation- the destruction of polyunsaturated lipids which leads to membrane damage and increased permeability.
Protein alteration- a process where polypeptide chains become fragmented leading to protein loss misfolding and alters protein protein interaction
DNA damage results in mutations
Mitochondrial effects- mitochondria and organelles that generate ATP. They can become damaged by ROS compromising available energy for the cell. Increase intercellular calcium also damage mitochondria.
Antioxidants
Molecules that inhibit the oxidation of other molecules thereby preventing the formation of free radicals. Often terminate a chain reaction that would result in radical formation. Ex: SOD, Catalase, Vit. C
Necrosis
Cellular consequence severe injury. Causes include ischemia, exposure to microbial toxins, chemical and physical agents, and rare example when active proteases leak out of cells and damage surrounding tissues. Characterized by ruptured plasma and lysosomal membrane, denaturation of cellular proteins, leakage of cellular contents, rapid loss of ATP and swelling of organelles, severe mitochondrial damage and local inflammation. Sum of cellular changes that occur in local cell injury.
Apoptosis
Active process of cellular self destruction resulting in programmed cell death.
Severe cell injury- when cell injury exceeds capacity for repair mechanisms cell signaling triggers this
Accumulation of misfolded proteins- this condition results from either genetic mutations or free radicals. leads to ER stress cumulates in cell death secondary to this.
Infections- may result from host immune response to presence of virus infecting cells. Cytotoxic T cells respond by inducing this thus eliminating infectious cells.
Obstruction of tissue ducts- obstruction of blood flow results in pathologic atrophy a process commonly noted in the pancreas kidney or parotid gland.