Cell Biology (Notes 37)
1/32
Earn XP
Description and Tags
Final Exam
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
What is an overview of carcinogenesis?
There are many ways to introduce mutations to DNA
Radiation
Chemicals
Infectious agents
Heredity
Those mutations will convert proto-oncogenes to oncogenes, and inactivates tumor suppressor genes
This leads to the instability of the genome
What is self-sufficiency?
Proliferation in the absence of growth factors
This phenomenon allows cancer cells to grow and divide independently, bypassing normal regulatory mechanisms that require external signals for cell growth
The graph:
X-axis: days of time in culture
Y-axis: cell number
The expectation is that the longer cells are in culture, the more they’ll grow
There are four lines on this graph:
Normal cells + serum growth factors: after blood clots, platelets in the blood release growth factors
Growth factors are important for the proliferation for the cells in culture
Exponential rise and then plateau
Normal cells - serum growth factors: a little growth, then plateau
Cancer cells + serum growth factors: more robust growth and division - continuous line/growth (exponential)
Cancer cells - serum growth factors: straight line
Cancer cells are able to growth in the absence of growth factors
Goes into a story of cell signaling
The signaling story:
Signal molecule causes receptor tyrosine kinase to autophosphorylate
Adaptor protein activates Ras activating protein, which activates Ras to be GTP bound
The onward signaling continues the cell cycle
All of this signaling is what you need to get the cell past the regulating events and get it to S phase
Cancer cells don’t pay attention to the regulating
Presence of growth factor regulates … cancer cells signal in the absence of growth factor
What are two examples of self-sufficiency?
Her2 receptor is mutated in about 30-35% of breast cancers:
Epidermal receptor that binds growth factor
In cancer, the protein being expressed by the proton-oncogene will develop a mutation that will lead to an amino acid substitution (oncogenic mutation)
Valine —> Glutamine
The mutation is in the transmembrane domain of the receptor spanning the plasma membrane
That mutation is sufficient to change the structure of the protein thus the receptors will auto-phosphorylate themselves in the complete absence of a growth factor
Once the mutation is acquired, Her2 becomes Neu, which is an oncoprotein
EGF receptor is a tyrosine kinase or a mutation that the protein that’s expressed in the cancer cell is a truncated protein, it lacks the extracellular domain —> no capacity to bind a growth factor—> renamed ErbB
Because it lacks an extracellular domain, it’ll go through a conformational change to allow the intracellular domain to undergo the process of autophosphorylation
What is insensitivity?
Insensitivity - antigrowth signals no longer recognized which results in release of G1 Arrest and degradation of ECM
There is a gene called p15 that encodes an inhibitor of cell cycle progression (this is a NORMAL cell)
If you are lacking p15, you’re going to continually go through cell divisions
There is another gene called PAI-1, which encodes an inhibitor of a protease that is needed to destroy the extracellular matrix
To keep the ECM intact, we can express an inhibitor of a destructive protease
In a normal cell, both genes must be expressed
Smad protein complex enters the nucleus and interacts with other promoters/transcription factors to regulate the two genes (p15 and PAI-1)
Smad becomes activated in response to receptors that are activated on the cell surface
These receptors are serine/threonine kinase receptors that can become phosphorylated
Those phosphorylation events are occurring in the intracellular domain
What is transforming growth factor beta (TGF beta)?
Growth factor that ensures the cells are NOT transformed
If there are mutations that interrupt this signaling event, the cell will start acquiring the characteristics of a cancer cell
A cell acquires mutations that prevents this signal from occurring - release of arrest in G1, cells enter the cell cycle, degradation of the ECM
What do cells need to do to stay healthy?
Have contact with the extracellular matrix
Experiment:
Placed glass squares of different dimensions in a medium and covered them in extracellular matrix proteins
On each protein, they placed a cell
On a graph:
X-axis - adhesive island area
Y-axis - apoptosis on the left
Y-axis - DNA synthesis on the right
Over time, the squares would experience higher amounts of DNA synthesis (cells)
Over time, the cells would experience lower apoptosis
How do you measure DNA synthesis?
Radioactive labeling
DNA probes
Measures increase or decrease of DNA content
How do you measure apoptosis?
Measure markers of apoptosis such as caspase activation, DNA fragmentation, and changes in mitochondrial membrane potential
What did they find from the experiment?
On the smaller adhesive islands, cells showed increased apoptosis and decreased DNA synthesis compared to larger islands, indicating that cell adhesion area directly influences cell survival and proliferation
cells require sufficient contact with the extracellular matrix for optimal function. Larger adhesive surfaces promote cell survival and growth, while smaller areas lead to increased cell death
On the smallest island, there is barely any cell growing
The conclusion of the experiment is that the cell’s contact with the ECM is a matter of life and death
If there is not sufficient contact with extracellular matrix, cell will undergo process of programmed cell death
Anoikis
Programmed cell death that is triggered by lack of contact with extracellular matrix
Important for excavation of tissues and prevention of tumors
What do the colors mean?
Red (ECM) - laminin
Green (apoptosis) - caspase
Blue - nuclei
What does each cell look like during anoikis?
On the right: the cell has no caspase (green):
The cells in the center are not dying - they’re going to keep multiplying
Lack of green (caspase)
Breast tumor
Bcl-2 levels are extremely high
With that, there is a potent inhibitor of programmed cell death
This is an escape from apoptosis (blocking apoptosis)
On the left: the cell has caspase (green):
The cells in the center are dying - not multiplying
Lack of green
Normal level of Bcl-2, which is putting a brake on programmed cell death
This is all happening in human mammary epithelial cells
What is evasion (escape of apoptosis)?
If there are high levels of MDM2, p53, a tumor suppressor gene, does not become activated
There is no expression of proteins needed for cell death
Expression of proteins at high levels can prevent cells from dying
What is immortality?
Limitless replicative potential
Stem cells can keep REPLICATING INDEFINITELY if the conditions are correct
The problem with cancer is that ALL THE CELLS have acquired immortality or a limitless replicative potential
These are all processes that are uncoupling a cell from mechanisms that are limiting cell proliferation — cell is uncoupled from natural process of programmed cell death
These uncoupling events do not ensure limitless replicative potential —> something else is happening
What does cancer-like immortality mean?
No limit as to how many times it can divide
Process of Immortality
While self-sufficiency, insensitivity, and evasion uncouple cells from mechanisms that limit proliferation but does not ensure LIMITLESS proliferation
Immortality allows limitless proliferation… but what prevents limitless proliferation?
Cell Lineages Undergo Replicative Senescence and Crisis
Has gone into G0 in cell cycle and is resting, but it has full potential to re-enter the cell cycle and go through more rounds of cell division
The graph:
X-axis: time (days after initiation of culture)
Y-axis: growth rate of culture
Certain cells experience a dip in growth rate - those cells are SENESCENT
Never re-enter the cell cycle
No growth happening in the culture
People look at the REPLICATIVE AGE of a cell (how many cell divisions has a cell gone through - each division is a birthday)
Hayflick said that when cell has gone through max number of divisions and gone into terminal G0 state, the cell has reached replicative limit
This is called the Hayflick Limit
These cells have a stable karyotype - the chromosomes are fine - the cell just won’t replicate
What drives cell senescence?
Metabolic stress on the physiology of the cell (accumulation of Reactive Oxygen Species - ROS)
ROS is causing molecular damage on the cell
In total, the damage is sensed by the cell and the cell isn’t moving forward anymore
What are the differences between normal and senescent cells?
Senescent cells are wider and more stretched out
What are crisis cells?
Defined by the VIABILITY of a cell
Viability of the culture CRASHES when cells are in crisis
Crisis is DIFFERENT from senescence
Crisis is where you have a lot of extreme instability of the genome
Unstable karyotypes - chromosomes are breaking and fusing together
Odd chromosomal morphologies - driven by the loss of the ends of the chromosomes or the telomeres
These cells will start dying and the loss of viability leads to apoptosis - the cell has damage that it cannot repair
There are many ways for a cell to die
Leading cause of cell death: apoptosis
Mitotic catastrophe - where chromosomes can’t segregate properly
What are the basics of DNA replication?
Origins of replication
Leading strands
Lagging strands - Okazaki fragments
There needs to be a primer for which DNA synthesis begins - that primer is a molecule of RNA that is annealing to the template strand
The polymerase recognizes the RNA primer and uses the template strand of DNA to synthesize the complementary strand of DNA
The RNA primer has to proceed where DNA synthesis will begin
However, what happens at the end of the chromosome?
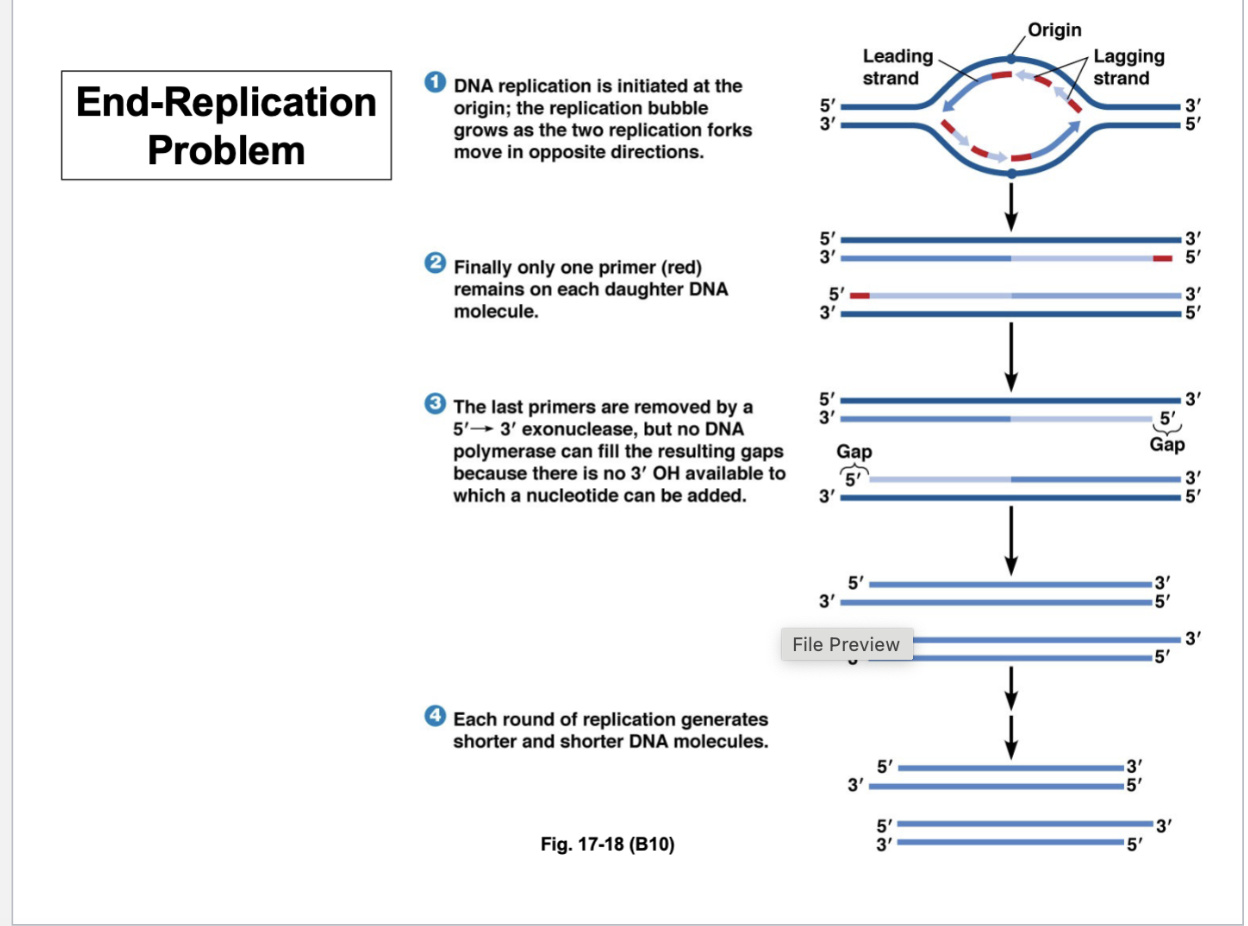
Telomeres
Primer is not covering the ends of the chromosome in the gap
Another primer is needed at the end of the gap to fill in the gap
After each round of replication, you’re always left with a gap at the end of the chromosome
Thus, the chromosome gets shorter and shorter with each generation
What happens to telomeres?
Ends of chromosomes are in red
As the ends of the chromosome shorten, the expression of certain genes are lost because they’re no longer there or half there
Those are the cells that are entering crisis because they no longer have a full complement of genes
With each generation, the telomeric DNA becomes shorter and shorter and lose the parts of the chromosome that have coding sequences
These cells enter crisis
How do the ends of chromosomes maintain their structure?
Ends of the chromosomes that are not functional - repetitive sequence
How do they produce telomeres in the absence of an RNA primer to allow DNA synthesis to synthesize the ends of the chromosomes?
The cell expresses telomerase
Telomerase
Binds a short RNA molecule (repetitive A’s and C’s)
That molecule serves as a template
That RNA gives information to the cell such that the telomerase can insert these corresponding complementary nucleotides (where there’s a C, there’s G at the 3’ end)
Telomerase is synthesizing the end of the chromosome and giving it the extra repetitive sequence
There’s still a gap - extend template strand
To synthesize missing DNA, all the normal methods of DNA replication occur - priming, DNA polymerase to fill in the gap
The telomeric sequences are repetitive, but they have this interesting ability to fold back on themselves and form a loop
Both ends of the chromosome have a loop of DNA - that’s how you finish the end of the chromosome
With each cell generation that these chromosomal ends become shorter and shorter and the cells enter crisis
What does this mean for the life of the cell and why does cell enter crisis?
Barbara McClintock
Through her work with maize genetics, she deduced that there is chromosomal breakage and fusion events occurring
Two broken chromosomes fuse together to form a new chromosome
This is especially important at the ends of the chromosomes
Fluorescent red chromosomes with the green dots at the end are visualized with FISH
Nucleic acid is used as a probe attached to another molecule
Every chromosome has a green dot - telomeric DNA is located here
Over each generation, the telomeres become shorter - they are formed early in life and telomerase shuts off —> ends of chromosome
With each cell division, chromosome ends become shorter
Chromosomes become too shorter —> crisis
Chromosomal instability drives crisis
Telomeres shown in red
Erode telomeres with each cell division - unprotected chromosomal ends
Those ends can fuse end to end — homologous chromosomes and sister chromatids and the ends are eroded
Arms will fuse with each other
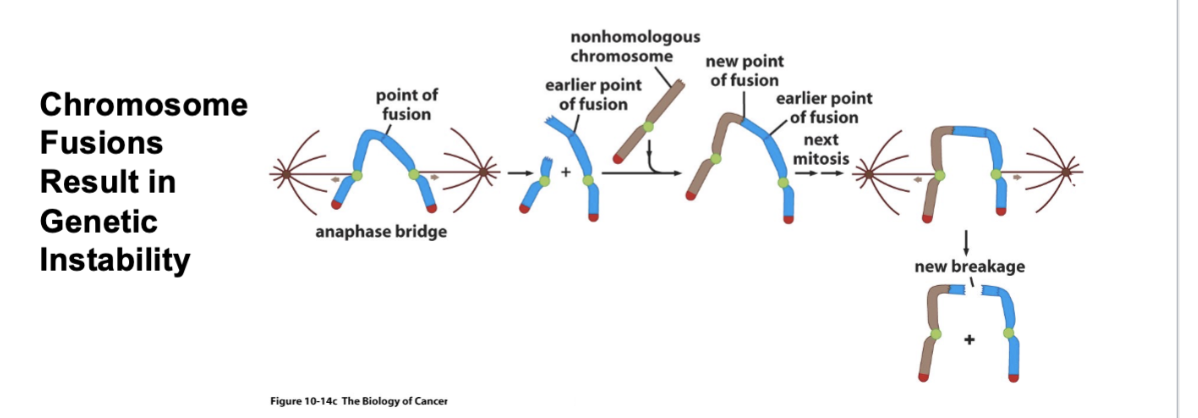
Where does the genetic instability come in?
Fused chromosomes in anaphase
Those kinetochores are the mitotic spindle — tension is being put on that odd chromosome
Because of the tension, there’s a short chromosome and a longer chromosome with an exposed end
Along comes a non-homologous chromosome —> brown chromosome will fuse with the blue chromosome
Next mitosis - new breakage
Eventually, the karyotype of the chromosomes will be a tangled mess of chromosomes
All of these bits of chromosomes are attached to each other
This is where the mechanism of programmed cell death kicks in and the cell dies from apoptosis
What is chronic myelogeneous leukemia?
Telomeric DNA is still there - but there is a breakage of chromosomes that leads to the development of cancer
Chromsomes 9 and 12 break and fuse
On Chromosome 9 - there’s ABL
On Chromsome 22 - there’s BCR
Breakage occurs about halfway through each gene - when two chromosomal fragments fuse together to form this small chromosome (Philadelphia chromosome)
Part of the BCR and the ABL gene are together - creating a chimeric gene
People don’t know WHY this leads to a cancerous state — ABL encodes a kinase and BCR encodes a GEF
We know this odd protein is highly expressed in cancer cells
Chromosomal translocations lead to the formation of novel fusion proteins that are hyperactive
This is a case where you have a proto-oncogene becoming an oncogene
What is Burkett’s lymphoma?
Chromosomal translocation results in active region of chromosome 14 in close proximity to the MYC gene of chromosome 8
These two chromosomes are translocating pieces —> leads to excess Myc protein
Myc is one of the early transcription factors needed for entering S phase - in excess, Myc will push cells into S phase
How do cancer cells escape crisis?
They escape crisis by expressing TELOMERASE (enzyme that builds the ends of the chromosomes)
Blackburn, Greider, Szostak found this discovery in a protist; McClintock also won a Nobel Prize on chromosome stability
The experiment:
X-axis: time (days)
Y-axis: culture growth (PDs)
At a certain point, growth of cell culture stops
HEK cells without hTERT has entered crisis
HEK cells with hTERT (telomerase) keeps increasing and increasing
Expressing telomerase is sufficient for allowing a cell to escape crisis
This is what is happening in cancer cells and allows them to keep replicating limitlessly
How does loss of telomerase trigger crisis and inhibit neoplastic growth?
There are four different cancer cell lines
Three lines per graph
Time (days) vs culture growth (PD)
Green lines show wild type level of telomerase (hTERT)
Blue line is the control
The red line is the mutated hTERT
Line functioning telomerase undergo VERY FEW DOUBLINGS
The expression of telomerase is ABSOLUTELY NECESSARY for the doubling of the cell population
Loss of telomerase triggers crisis and inhibits neoplastic and cancerous growth of cells
Summarizing Telomeres
Somatic cells have reduced telomeric DNA sequences because with each cell division, you lose a little bit more of the chromosome
Eventually, once you lose your last telomeric DNA sequence, those cells enter crisis
Cancer cells expressing telomerase have very long telomeres - those cells will keep growing over time, yet those cells can display genetic instabilities where there are breakages and translocations on the length of chromosomes
Not at end because ends are protected
Stem cells also have long telomeres so they can divide limitlessly
Embryonic cells also have long telomeres, the starting point of our DNA
Why do we have long telomeres in embryonic but not somatic cells?
Because adult cells are NOT expressing telomerase
But embryonic cells are expressing telomerase because they are building telomeres to generate a population of 37 trillion cells