SCH4U - U2 Redox Reactions
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
64 Terms
1. The oxidation number of chromium ( ) in the dichromate ion Cr2O72- is
a.+2 | b.+4 | c.+6 | d.+7 | e. -2 |
c.+6
2. The oxidation number of oxygen in ozone, O3, is
a.+3 | b.3 | c.+2 | d.2 | e.0 |
e.0
3. In Fe2(SO4)3 the oxidation numbers of and respectively are
a. | +2, +3, 4 | c. | +2, +4, 8 | e.+4, +5, 3 |
b. | +3, +6, -2 | d. | +2, +4, -2 |
b. | +3, +6, -2 |
In the reaction
2MnO4-(aq) + 5C2O42-(aq)+ 16H+(aq) → 2Mn2+(aq) + 10CO2(g) + 8H2O (l)
Which of the following statements is correct?
a. | hydrogen is oxidized and C2O42- is the oxidizing agent | d. manganese is oxidized and C2O42- is the oxidizing agent |
b. | hydrogen is oxidized and MnO41- is the oxidizing agent | e. carbon is oxidized and MnO41- is the oxidizing agent |
c. | carbon is oxidized and H+ is the oxidizing agent |
e. carbon is oxidized and MnO41- is the oxidizing agent |
determine whats LEO and GER using oxidation numbers
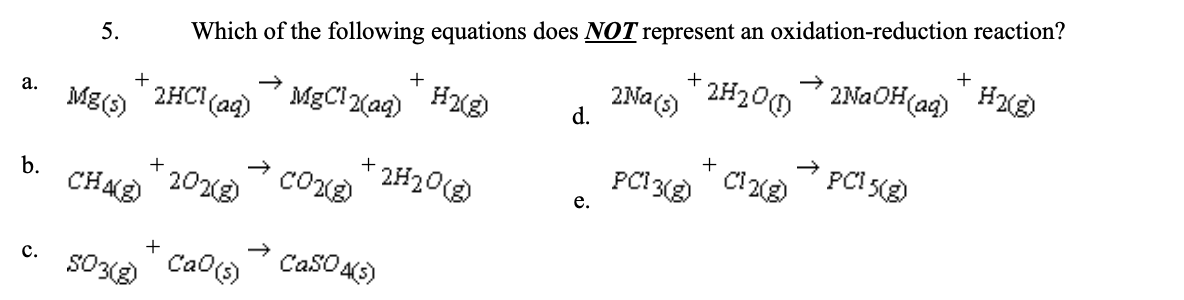
5. Which of the following equations does NOT represent an oxidation-reduction reaction?
c. SO3(s) + CaO(s). → CaSO4(s)
6. Consider the following oxidation-reduction reaction:
KIO3 + 5KI + 6HCl → 2I2 + 3H2O + 6KCl
Which of the following statements about this oxidation-reduction process is true?
a. | iodine is oxidized and potassium is reduced | d. chlorine is oxidized and hydrogen is reduced |
b. | iodine is reduced and potassium is oxidized | e. iodine is both oxidized and reduced |
c. | potassium is oxidized and chlorine is reduced |
e. iodine is both oxidized and reduced
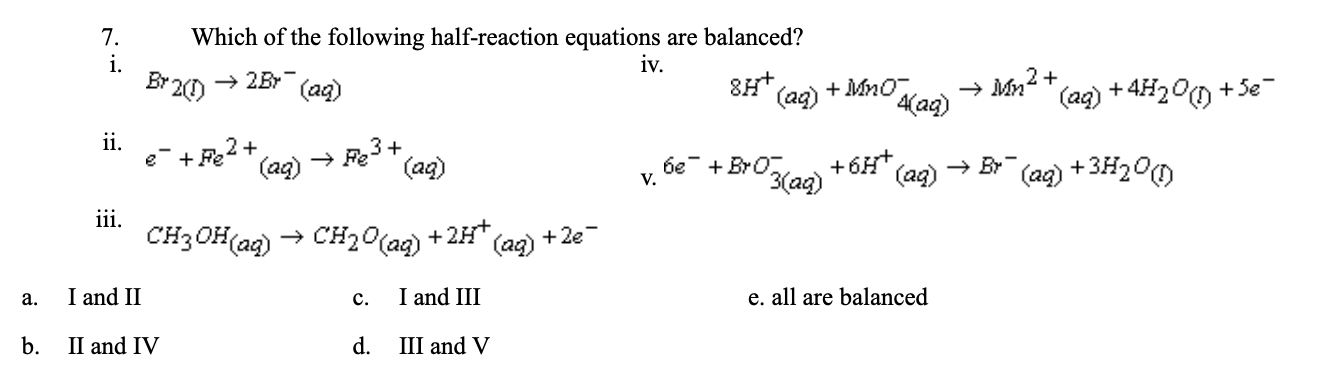
7.
d.III and V
CH3OH (aq) → CH2O(aq) + 2H+ + 2E-
6e- + BrO3- (aq) + 6H+(aq) → Br- (aq) + 3H2O(l)
8. Which of the following statements are true for all electric cells?
i. electrons flow from the anode to the cathode
ii. reduction occurs at the anode
iii. oxidation occurs at the cathode
iv. oxidation occurs at the anode
v. reduction occurs at the cathode
a. | I, IV and V | c. | II and III | e. | I and III |
b. | I, II and III | d. | IV and V | ||
a. | I, IV and V |
i. electrons flow from the anode to the cathode
iv. oxidation occurs at the anode
v. reduction occurs at the cathode
9. The following two half-reactions are involved in a galvanic cell:
Cu2+ + 2e- → Cu, Eo= +0.34V
Zn2+ + 2e- → Zn, Eo = -0.76V
At standard conditions, what species are produced at each electrode?
a. | Cu is produced at the cathode and Zn at the anode | d. Cu2+ is produced at the cathode and Zn at the anode |
b. | Cu is produced at the cathode and Zn2+ at the anode | e. Cu2+ is produced at the cathode and Zn2+ at the anode |
c. | Cu2+ is produced at the anode and Zn at the cathode |
b. | Cu is produced at the cathode and Zn2+ at the anode |
draw a galvanic cell showing this to help you visualize
10. An electrochemical cell is set up with a copper electrode in contact with 1.0 mol/L CuSO4(aq) and a lead electrode in contact with 1.0 mol/L Pb(NO3)2(aq). The standard reduction potentials are
Pb2+ + 2e- → Pb, Eo= -0.13V
Cu2+ + 2e- → Cu, Eo= +0.34V
At 25 deg C, the standard cell potential for this galvanic cell in volts is
a. | 0.13 | b. | 0.34 | c. | 0.47 | d. | 0.21 | e. | 0.94 |
c. | 0.47 |
Given:
Pb²⁺ + 2e⁻ → Pb, E° = –0.13 V
Cu²⁺ + 2e⁻ → Cu, E° = +0.34 V
Step 1: Identify cathode and anode
The cathode is where reduction happens (higher E°).
The anode is where oxidation happens (lower E°).
Since +0.34 V (Cu²⁺/Cu) is greater than –0.13 V (Pb²⁺/Pb):
Cathode: Cu²⁺ + 2e⁻ → Cu
Anode: Pb → Pb²⁺ + 2e⁻ (oxidation is the reverse of the Pb²⁺ reduction)
Step 2: Calculate standard cell potential
E°cell = E°cathode – E°anode
E°cell = (+0.34 V) – (–0.13 V)
E°cell = +0.34 V + 0.13 V
E°cell = +0.47 V
Given the following half-cell reactions:
Fe2+(aq) + 2e- → Fe(s), Eo = -0.44V
Cr3+(aq) + 3e- → Cr(s), Eo= -0.74V
At 25oC, the initial cell voltage of the cell shown in the diagram above is
a.1.18 V | b.-0.30 V | 1.18 V | d.+0.30 V | e.1.34 V |
d.+0.30 V
Given half-reactions:
Fe²⁺ + 2e⁻ → Fe, E° = –0.44 V
Cr³⁺ + 3e⁻ → Cr, E° = –0.74 V
Step 1: Identify cathode and anode
The cathode is where reduction happens — choose the half-reaction with the higher (less negative) E°.
Fe²⁺/Fe: E° = –0.44 V → this is the cathode (better at being reduced)
Cr³⁺/Cr: E° = –0.74 V → this is the anode (will be oxidized, reverse of reduction reaction)
Step 2: Calculate standard cell potential
E°cell = E°cathode – E°anode
E°cell = (–0.44 V) – (–0.74 V)
E°cell = –0.44 V + 0.74 V
E°cell = +0.30 V
12. Which of the following statements are true?
i. Aluminum is protected from corrosion by the formation of a thin layer of aluminum oxide on the surface.
ii. Iron is protected from corrosion by the formation of a layer of iron oxide on the surface.
iii. Cathodic protection of iron uses a metal that is more easily reduced than iron.
iv. Cathodic protection of iron uses a metal that is more easily oxidized than iron.
a. | I and II | c. | I and IV | e. | II and IV |
b. | I and III | d. | II and III | ||
b. | I and III |
13. What us the purpose of the salt bridge in an electrochemical cell?
a. | To maintain electrical neutrality in the half-cells via migration of ions. |
b. | To provide a source of ions to react at the anode and cathode. |
c. | To provide oxygen to facilitate oxidation at the anode. |
d. | To provide a means for electrons to travel from the anode to the cathode. |
e. | To provide a means for electrons to travel from the cathode to the anode. |
a. | To maintain electrical neutrality in the half-cells via migration of ions. |
14. Which of the following is the strongest oxidizing agent?
a.H202(aq) | b.Fe3+(aq) | c.ClO2(g) | d.I2(s) | e.Fe(s) |
a.H202(aq)
15. What statement is true about standard electrode potentials?
i. E°cell is positive for spontaneous reactions.
ii. Electrons will flow from more negative electrode to more positive electrode.
iii. The electrode potential of the standard hydrogen electrode is exactly zero.
a. | i.) only | c. | i.) and ii.) only | e. | All of i.), ii.) and iii.) |
b. | i.) and iii.) only | d. | ii.) and iii.) only | ||
e. | All of i.), ii.) and iii.) |
*1. The spectator ion in the equation Al(NO3)3(aq) + Zn(s) → Al(s) + Zn(NO3)(aq) is
a. Al3+
b.Zn2+
c. Al3+ and Zn2+
d. Al and Zn
e.NO3
e.NO3
*2. What is the correct balanced net ionic equation for the reaction: Fe(NO3)2(aq) + Ni(s) → Fe(s) + Ni(NO3)2(aq)
a. 2Fe2+ (aq) +3Ni(s) →2Fe(s) +3Ni2+(aq)
b. 3Fe2+(aq) + 2Ni(s) → 3Fe(s) +2Ni2+(aq)
c. Fe2+(aq) + Ni(s) → Fe(s) + Ni2+(aq)
d. Fe(NO3)2(aq) + Ni(s) → Fe(s) + Ni(NO3)2(aq)
e. 2Fe(NO3)2(aq) + 3Ni(s) → 2Fe(s) + 3Ni(NO3)2(aq)
c. Fe2+(aq) + Ni(s) → Fe(s) + Ni2+(aq)
A net ionic equation focuses on the chemical reaction by including only the substances that directly participate in the reaction, omitting spectator ions that don't change… aka “What’s cool”
*3. Use this reaction for the following 2 questions.
Cr(NO3)3 (aq) +Mg(s) →Cr (s) +Mg(NO3)2 (aq)
In the reaction above which element loses electrons in the process
a. Chromium
b. nitrogen
c. magnesium
d. oxygen
e. none of the above lose electrons
c. magnesium
look at oxidation numbers
Cr(NO3)3 (aq) +Mg(s) →Cr (s) +Mg(NO3)2 (aq)
*4. In the reaction above which element gains electrons in the process
a. Chromium
b. nitrogen
c. magnesium
d. oxygen
e. none of the above gain electrons
a. Chromium
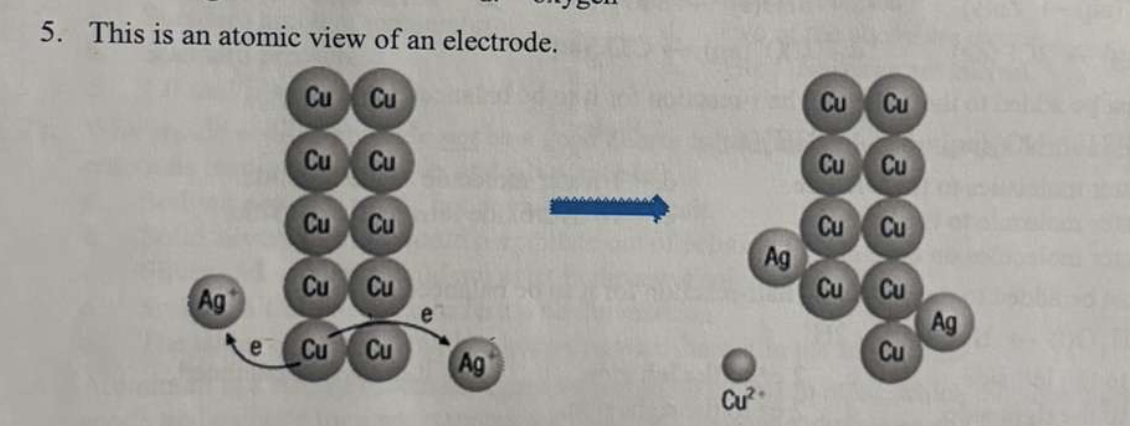
*5. The image shows an atomic view of an electrode. Fill in the statement:
Copper is ______ while silver is _____
a. reduced; oxidized
b. oxidized; reduced
c. none of the above
b. oxidized; reduced
*6. Identify the oxidizing agent in the reaction: 2Fe(s) + O2(g) + 2H2O(l) → 2Fe(OH)2(s)
a. Fe(s)
b. H2O(l)
c. O2(g)
d. Fe and O2
e. Fe(OH)2
c. O2(g)
*7. In the unbalanced equation Al(s) + Cl2(g) → AlCl3(s)
a. aluminum chloride is the oxidizing agent
b. aluminum is the oxidizing agent
c. chlorine is the oxidizing agent
d.chlorine is the reducing agent
d.chlorine is the reducing agent
*8. In the reaction KNO3(aq) + PbSO4(aq) → Pb(NO3)2(aq) + K2SO4(aq) What is the oxidizing agent
a. KNO3(aq)
b. PbSO4 (aq)
c. Pb(NO3)2 (aq)
d. K2(SO4)(aq)
e. there are none
e. there are none
*9. The oxidation number of phosphorus in P4 is
a. 0
b. +4
c. -4
d. +1/4
e. -1/4
a. 0
*10. What is the oxidation number of zinc in Zn2+(aq)
a. 0
b. +2
c. -2
d. +1
e. -1
b. +2
*11. What is the oxidation number of the oxygen atoms in H2O2(s)
a. 0
b. -1/2
c. -1
d. -2
e. -3
c. -1
*12. What is the oxidation number of N in NH4+(aq)
a. +1
b. +3
c. -3
d. +4
e. - 4
c. -3
*13. What is the oxidation number of a carbon atom in C4H8O
a. 0
b. +3/2
c. -3/2
d. +6
e. -6
c. -3/2
*14. If an element goes from an oxidation number of +2 to an oxidation number of -2, it is an
a. oxidizing agent
b. reducing agent
c. spectator ion
d. reduced then oxidized
e. oxidized then reduced
a. oxidizing agent
*15. Which of the following represents a reduction half reaction?
a. H2(g) → H2O (l)
b. NO2(g) → NH3 (aq)
c. Cl-(aq) → ClO3-(aq)
d. H2AsO4-(aq) → As2S3(s)
e. Cd(s) -? Cd2+ (aq)
b. NO2(g) → NH3 (aq)
*16. Which of the following representss an oxidation half reaction
a. Zn2+ (aq) → Zn(s)
b. Cl2 (g) →2CI-(aq)
c. SnO(s) → SnO2(s)
d. ClO3 - → ClO2-(aq)
e. All of the above
c. SnO(s) → SnO2(s)
*17. What must be added to the following half-reaction for it to be balanced?
10H+ +NO3- (aq) +8e- → NH4+(aq)
a. 3 water molecules to the left side
b. 1 water molecule to the right side
c. 3 water molecules on the right side
d. 1 water molecule to the left side
e. 10 hydroxide ions to the left side
c. 3 water molecules on the right side
*18. What must be added to the following half-reaction for it to be balanced?
2H20(l) → H2O2 (aq) +2H+
a. 1 e to the left side.
b.1 e to the right side.
c. 2 e to the left side.
d. 2 e to the right side.
e. it is already balanced
d. 2 e to the right side.
*19. How many hydrogen ions must be added to the half-reaction to balance the mass of hydrogen atoms?
CN- (aq) → CNO- (s)
a. 1 on the left side.
b. 1 on the right side.
c. 2 on the left side.
d. 2 on the right side.
e. No hydrogen ions are needed.
d. 2 on the right side.
To balance oxygen, we:
Add H₂O to the side missing oxygen
Then add H⁺ to balance the hydrogens introduced from H₂O
Let’s do that:
Unbalanced:
CN⁻ → CNO⁻
We need 1 O on the left, so we add H₂O:
Balanced for O:
H₂O + CN⁻ → CNO⁻
Now we have:
2 H atoms on the left
None on the right
So, to balance hydrogen, we add 2 H⁺ to the right side:
Final mass-balanced half-reaction:
H₂O + CN⁻ → CNO⁻ + 2H⁺
*20. Which of the following reactions is an example of a disproportionation reaction?
a. PbCl2(s) _> Pb(s) + Cl2,(g)
b. K(s) + Cl2(g) → KCl (aq)
c. Fe(s) + CuSO4(aq) -> FeSO4(aq) + Cu (s)
d. NO3-(aq) + NH3(g) → 2NO2-(s)
e. 3Cl2 (g) +6OH- (aq) →5CI- (aq) +CIO3-(aq) +3Н20(l)
e. 3Cl2 (g) +6OH- (aq) →5CI- (aq) +CIO3-(aq) +3Н20(l)
The same element is both oxidized and reduced at the same time.
Start: Cl₂ (0)
End:
Cl⁻ (–1) → reduction
ClO₃⁻ (Cl is +5) → oxidation
✅ The same element (Cl) is both oxidized (0 → +5) and reduced (0 → –1)
*21. If the following reaction is balanced, how many electrons are transferred?
Fe2+(aq) + Al (s) → Al3+(aq) + Fe(s)
a. 2
b. 3
c.4
d. 5
e. 6
e. 6
*22. Which electrode is the site of reduction
a. Inert anode
b. the salt bridge
e. the anode
d. the cathode
d. the cathode
*23. Which of the following statements is correct for an electrochemical cell?
a. The anode and cathode both increase in mass.
b. The anode and cathode both decrease in mass.
c. The anode increases in mass while the cathode decreases in mass.
d. The anode decreases in mass while the cathode increases in mass.
e. There is never a change in mass for either electrode in an electrochemical cell.
d. The anode decreases in mass while the cathode increases in mass.
*24. Identify the anode in the following reaction.
Zn(s) + SnSO4(aq) → ZnSO4(aq) + Sn(s)
a. Zn (s)
b. SnSO4
c, ZnSO4
d, Sn(s)
e, not a redox reaction
a. Zn (s)
*25. What is the standard half cell potential for the hydrogen half ceell
a. 0.00V
b. +1.00V
c. -1.00V
d. +0.10V
e. -0.10V
a. 0.00V
*26. What does tthe word “standard” refer to in standard half cell potential
a. standard ambient temperature
b. standard presssure
c. 1.0mol/L solutions
d. two of the above are correct
e. all of the above are correct
e. all of the above are correct
*27. Why would sodium chloride not be a good choice for the electrolyte in the salt bridge if one of the half reactions involved solid silver and silver nitrate?
a. Sodium chloride would not complete the circuit.
b. Solid silver chloride would precipitate out of solution.
c. Silver and sodium should not exist in the same solution together.
d, Sodium nitrate would interfere with the reaction.
e, The silver electrode would be corroded with the salt in the salt bridge.
b. Solid silver chloride would precipitate out of solution.
*28. Aluminum is a stronger reducing agent than zinc. With this in mind, which metals would be used as the anode and cathode for a spontaneous galvanic cell involving aluminum and zinc?
a, A platinum anode and a zine cathode.
b. An aluminum anode and a platinum cathode.
c. An aluminum anode and a zinc cathode.
d. A zine anode and an aluminum cathode.
e. The cathode and anode would both be platinum.
c. An aluminum anode and a zinc cathode.
*29. Which of the following is a stronger reducing agent than Mg(s)
a. Ca(s)
b. Pt(s)
c. Sn(s)
d. Fe(s)
e. Cu(s)
a. Ca(s)
*30. Which of the following is a stronger oxidizing agent than Fe²⁺(aq)?
a. Cr³⁺(aq)
b. Cd²⁺(aq)
c. Zn²⁺(aq)
d. Al³⁺(aq)
e. Mg²⁺(aq)
b. Cd²⁺(aq)
31. In the reaction
Fe(s) + O₂(g) → Fe₂O₃(s)
which element gains electrons in the process?
a. Iron
b. Oxygen
c. Iron and oxygen
d. Iron (III) oxide
e. None of them.
b. Oxygen
*32. In the list below, which metal or ion is the strongest reducing agent?
A Short Table of Reduction Potentials of Unknown Metals
W³⁺(aq) + 3e⁻ ⇌ W(s), E° = +1.50 V
X²⁺(aq) + 2e⁻ ⇌ X(s), E° = –0.14 V
Y²⁺(aq) + 2e⁻ ⇌ Y(s), E° = –0.28 V
Z²⁺(aq) + 2e⁻ ⇌ Z(s), E° = –2.76 V
a. W³⁺ ion
b. W metal
c. Z²⁺ ion
d. Z metal
d. Z metal
Strongest reducing agent = the species that most easily loses electrons (gets oxidized).
Since the table gives reduction potentials (E°), the lower (more negative) the reduction potential, the more easily the reverse (oxidation) reaction happens → stronger reducing agent.
33. Which of these reactions will proceed spontaneously, based on relative strength of reducing agents?
a. Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s)
b. Sn(s) + Cr³⁺(aq) → Sn²⁺(aq) + Cr(s)
c. Cd(s) + Al³⁺(aq) → Cd²⁺(aq) + Al(s)
d. Ag(s) + Zn²⁺(aq) → Ag⁺(aq) + Zn(s)
e. Co(s) + Fe²⁺(aq) → Co²⁺(aq) + Fe(s)
a. Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s)
A reaction is spontaneous if the metal that is oxidized (the reducing agent) is stronger than the metal ion that is reduced (the oxidizing agent).
34. An electrochemical cell is set up with a copper electrode in contact with 1.0 mol/L CuSO₄(aq) and a lead electrode in contact with 1.0 mol/L Pb(NO₃)₂(aq). The standard reduction potentials are:
Pb²⁺ + 2e⁻ → Pb; E° = –0.13 V
Cu²⁺ + 2e⁻ → Cu; E° = +0.34 V
At 25°C, the standard cell potential for this galvanic cell in volts is:
a. 0.13
b. 0.34
c. 0.47
d. 0.21
e. 0.94
c. 0.47
We’re given standard reduction potentials:
Cu²⁺ + 2e⁻ → Cu E° = +0.34 V
Pb²⁺ + 2e⁻ → Pb E° = –0.13 V
👉 The more positive E° is favored for reduction.
✅ Cu²⁺ is reduced at the cathode.
✅ Pb is oxidized at the anode.
2⃣ Calculate the cell potential
Standard cell potential:
E°cell = E°cathode – E°anode
E°cathode = +0.34 V (Cu²⁺/Cu)
E°anode = –0.13 V (Pb²⁺/Pb)
So:
E°cell = 0.34 V – (–0.13 V) = 0.34 V + 0.13 V = 0.47 V
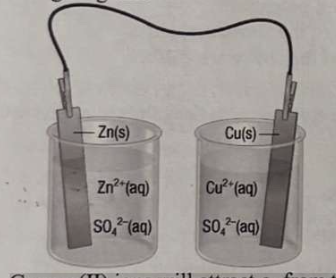
35. Which statement correctly describes what will happen when the half-cells are connected as shown in the following diagram?
a. Copper(II) ions will attract e⁻ from the zinc electrode, and current will flow indefinitely.
b. Current will flow for an instant, then stop.
c. Current will not flow at all.
d. Zinc ions will attract e⁻ from the copper electrode, and current will flow indefinitely.
b. Current will flow for an instant, then stop.
1. Explain the significance of the standard hydrogen electrode (SHE) in the tabulation of standard reduction potentials of other species. (3 marks)
The Standard Hydrogen Electrode (SHE) is 2H+ + 2e- → H2 and is used as a reference to construct electrochemical cells with other species. By setting the SHE's standard reduction potential to 0.00 V, the standard cell potential (E°cell) can be measured and used to calculate the unknown species’ standard reduction potential using the equation:
E°cell = E°unknown – E°SHE.
Since E°SHE is zero, this simplifies to E°cell = E°unknown. In this way, the entire table of standard reduction potentials is built. A species with a positive E°red is more easily reduced than hydrogen, while one with a negative E°red is more easily oxidized.
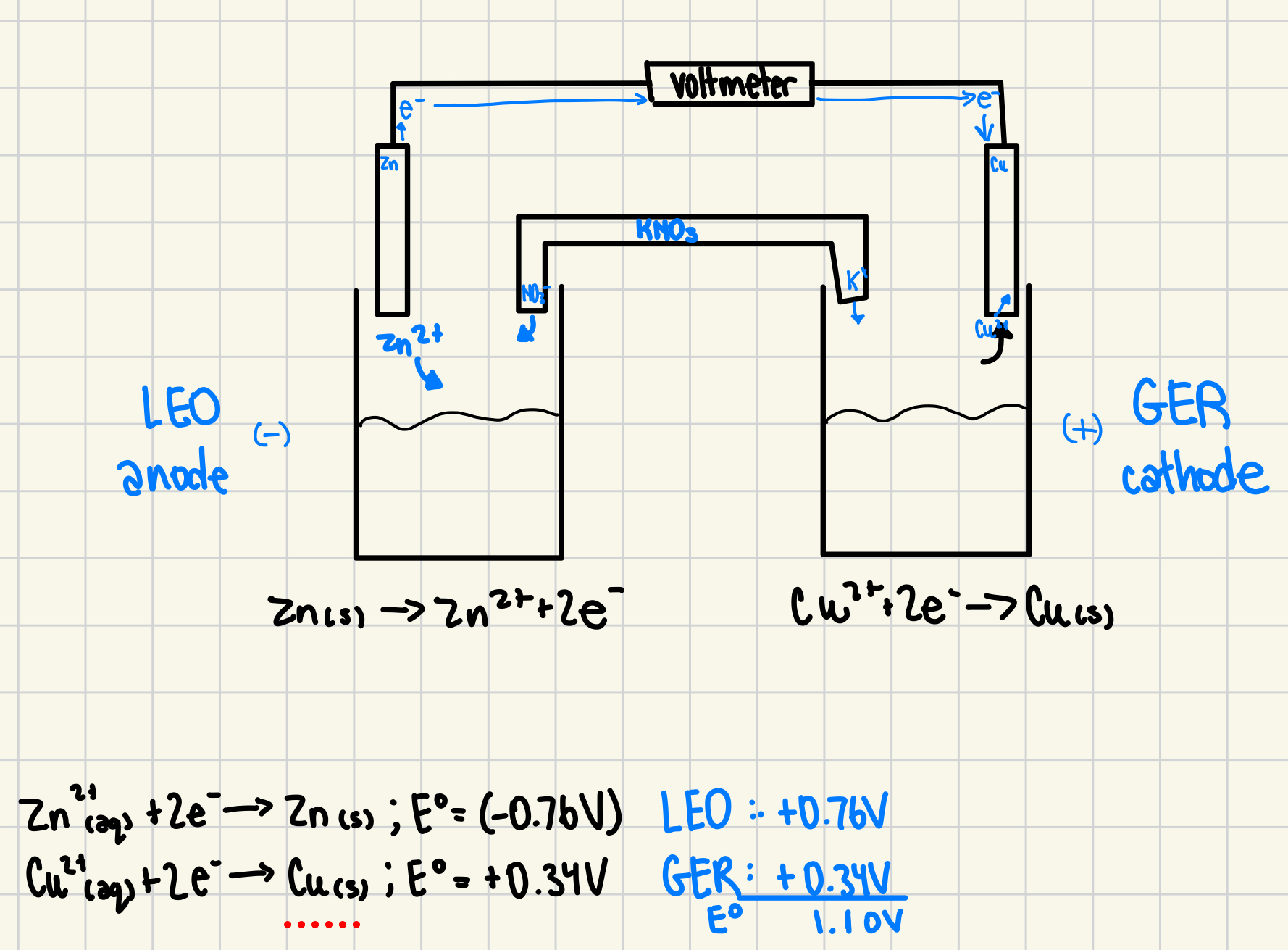
2. A voltaic cell is constructed using solid zinc in zinc nitrate and solid copper in copper nitrate with a potassium nitrate salt bridge. Sketch the voltaic cell that contains the following half reactions. Label all relevant components including anode and cathode. Use arrows to show: the movement of electrons through the wire, movement of ions in the salt bridge, and movement of ions in the half-cells. (5 marks)
Zn2+(aq) + 2e- → Zn(s) Eo = (-0.76 V)
Cu2+(aq) + 2e- → Cu(s) Eo = +0.34 V
3. USE THE HALF-REACTION METHOD and balance the following equation occurring in basic solution: (6 marks)
I-(aq) + MnO4-(aq) → I2(aq) + MnO2(s) (basic)
3. 4H2O + 6I- + 2MnO4- → 2MnO2 + 3I2 + 8OH-

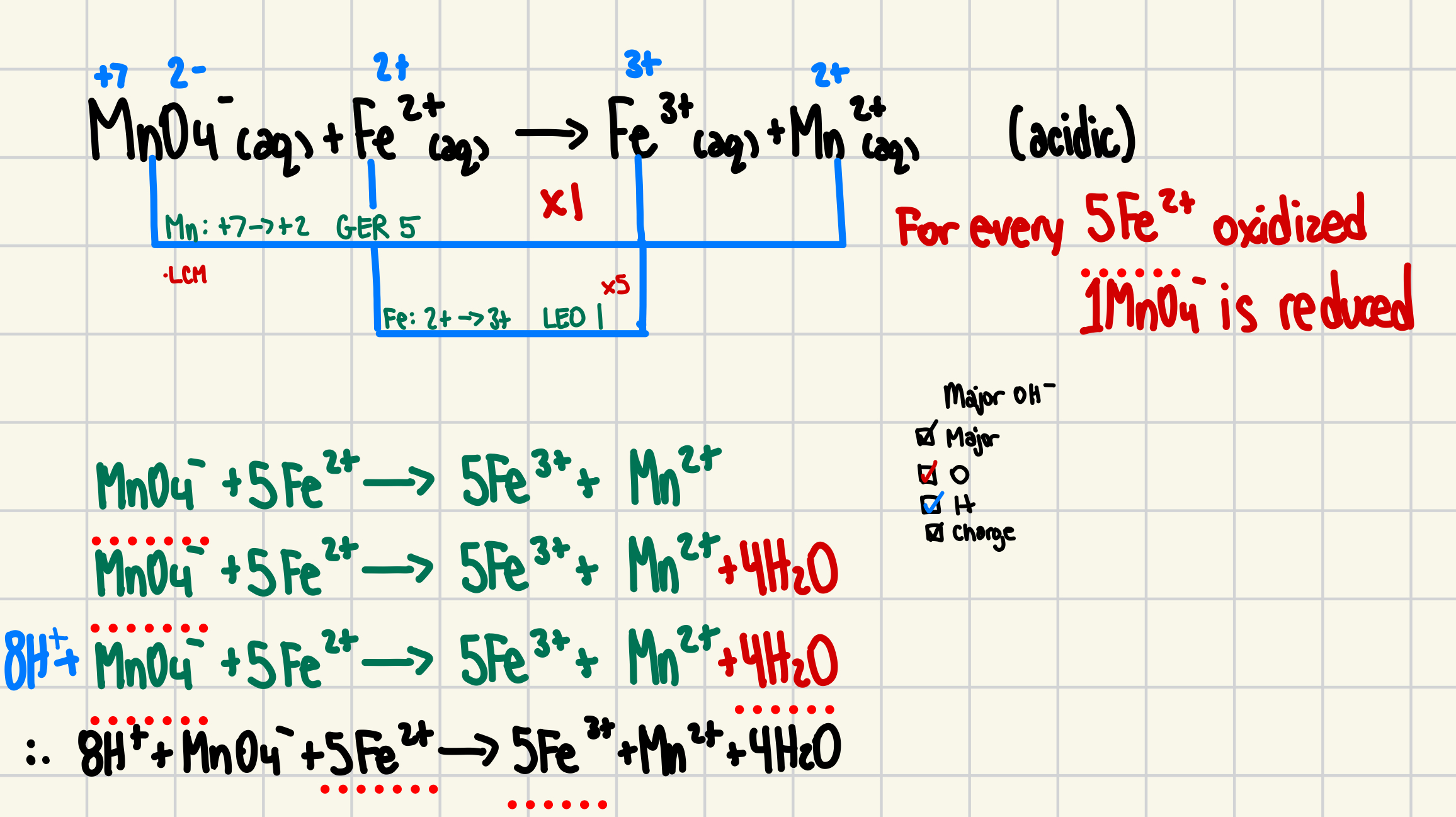
4. For the following, identify the redox participants (what is oxidized, what is reduced) and SHOW all steps in balancing USING THE OXIDATION NUMBER METHOD:
In acidic solution, permanganate ions are used to analyze iron content in ores (6 marks)
MnO4-(aq) + Fe2+(aq) → Fe3+(aq) + Mn2+(aq) (acidic)
4. MnO4-1 + 5Fe2+ + 8H+ → 5Fe3+ + Mn2+ + 4H2O
dont forget states
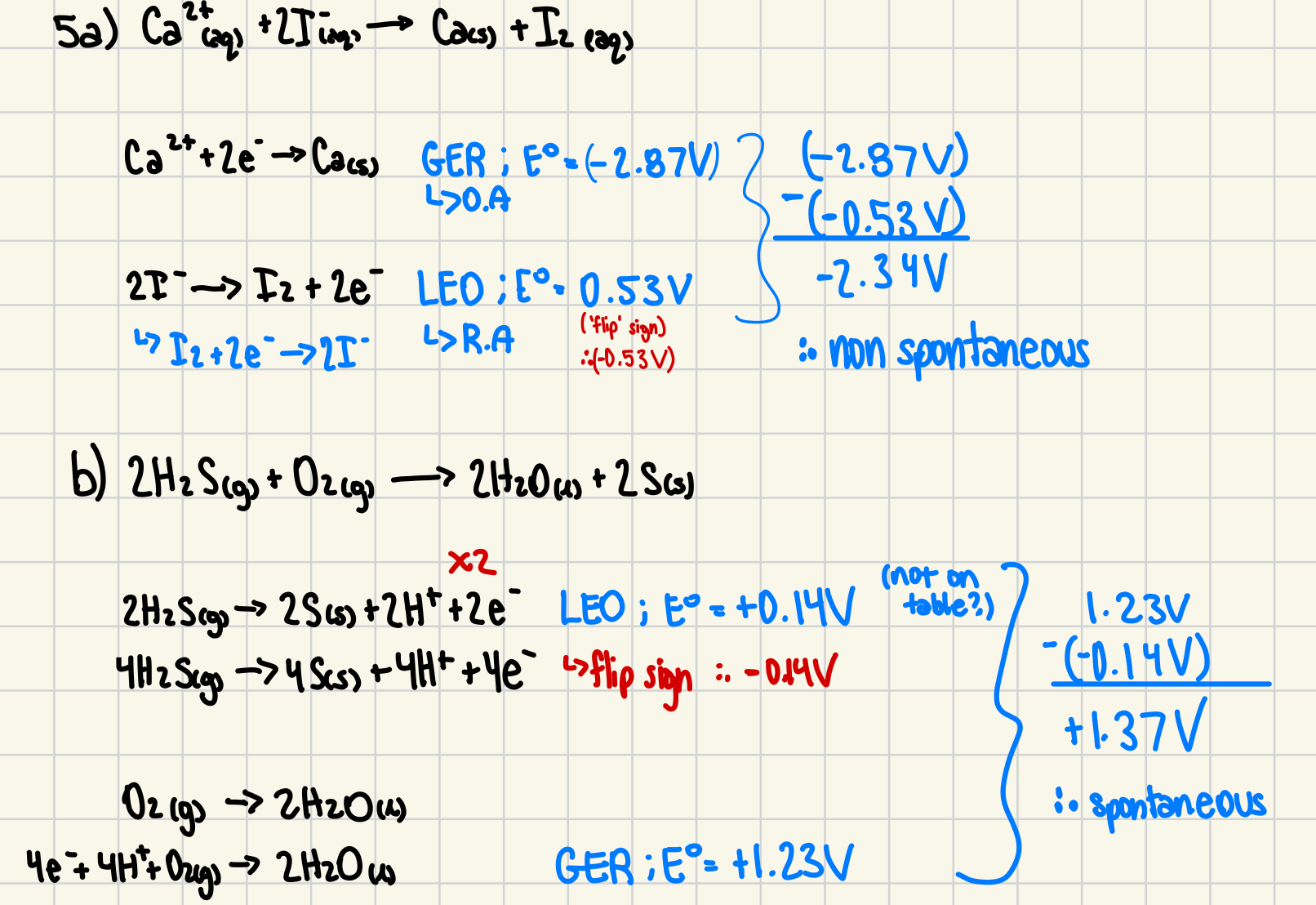
5. Which of the following reactions will be spontaneous as written? Explain (or show) why.
a) Ca2+(aq) + 2 I-(aq) → Ca(s) + I2(aq)
b) 2 H2S(g) + O2(g) → 2 H2O(l) + 2 S(s)
6. Construct and fully label a voltaic cell from the following reduction reactions: (ID the anode/cathode) and provide Eocell
Mg2+(aq) + 2e- → Mg(s)
Cu2+(aq) + 2e- → Cu(s)
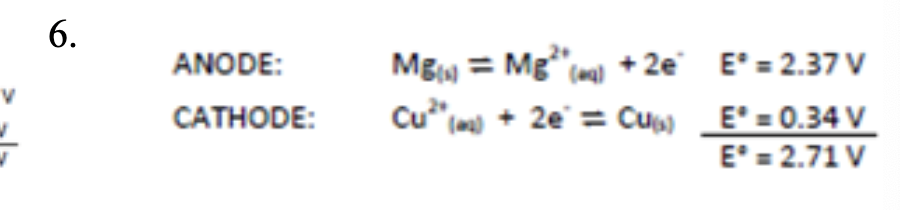
1. Fully explain why the following reaction will NOT occur. (3 marks)
Sn²⁺(aq) + Pb⁴⁺(aq) → Sn(s) + Pb²⁺(aq)
Sn²⁺ → Sn(s): Tin is going from +2 to 0 → being reduced (gaining electrons, GER).
Pb⁴⁺ → Pb²⁺: Lead is going from +4 to +2 → being reduced (gaining electrons, GER).
The reaction
Sn²⁺(aq) + Pb⁴⁺(aq) → Sn(s) + Pb²⁺(aq)
will not occur because Both Sn²⁺ and Pb⁴⁺ are gaining electrons (getting reduced), but a redox reaction requires one species to be oxidized and one to be reduced. Since no species is being oxidized, the reaction cannot proceed. Alongside this, the total charge on the reactant side is +6 (Sn²⁺ + Pb⁴⁺), while the product side is only +2 (Pb²⁺ + Sn(s)). Because charge is not conserved, the reaction is not balanced and cannot occur.
2. Use the oxidation number method to balance the following redox reaction in basic conditions. (6 marks)
Sn2+(aq) + Cr2O72-(aq) → Sn4+(aq) + Cr3+(aq)
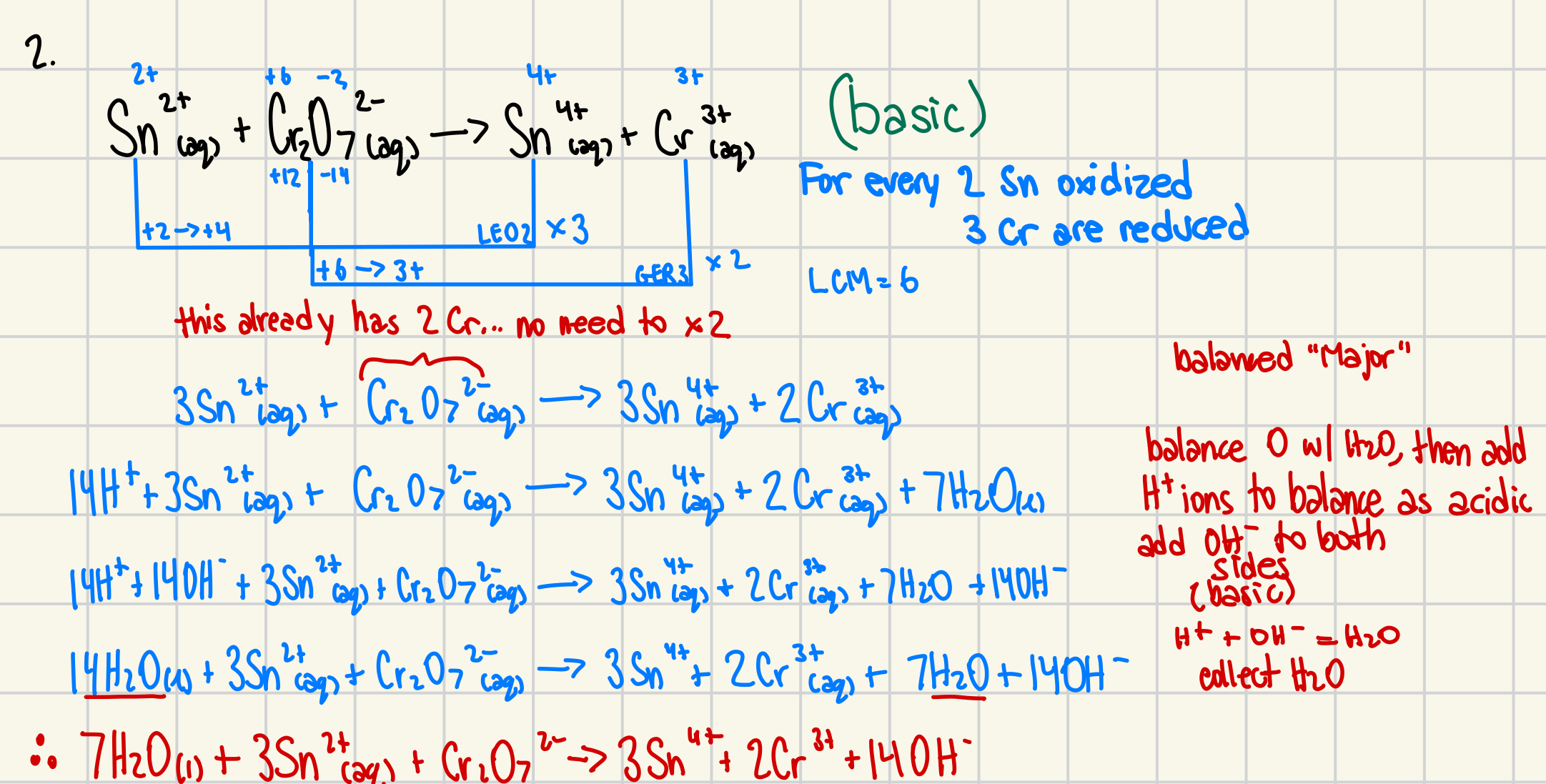
3. Balance each redox reaction in acidic conditions using the half-reaction method. (6 marks)
ClO3-(aq) + I2(s) → IO3-(aq)+ Cl-(aq)

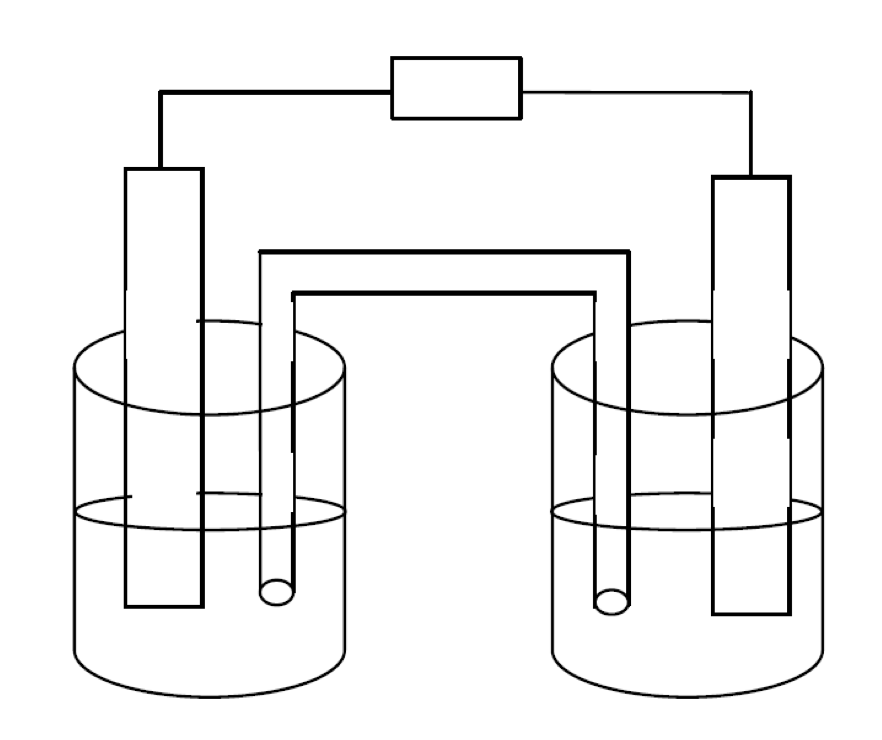
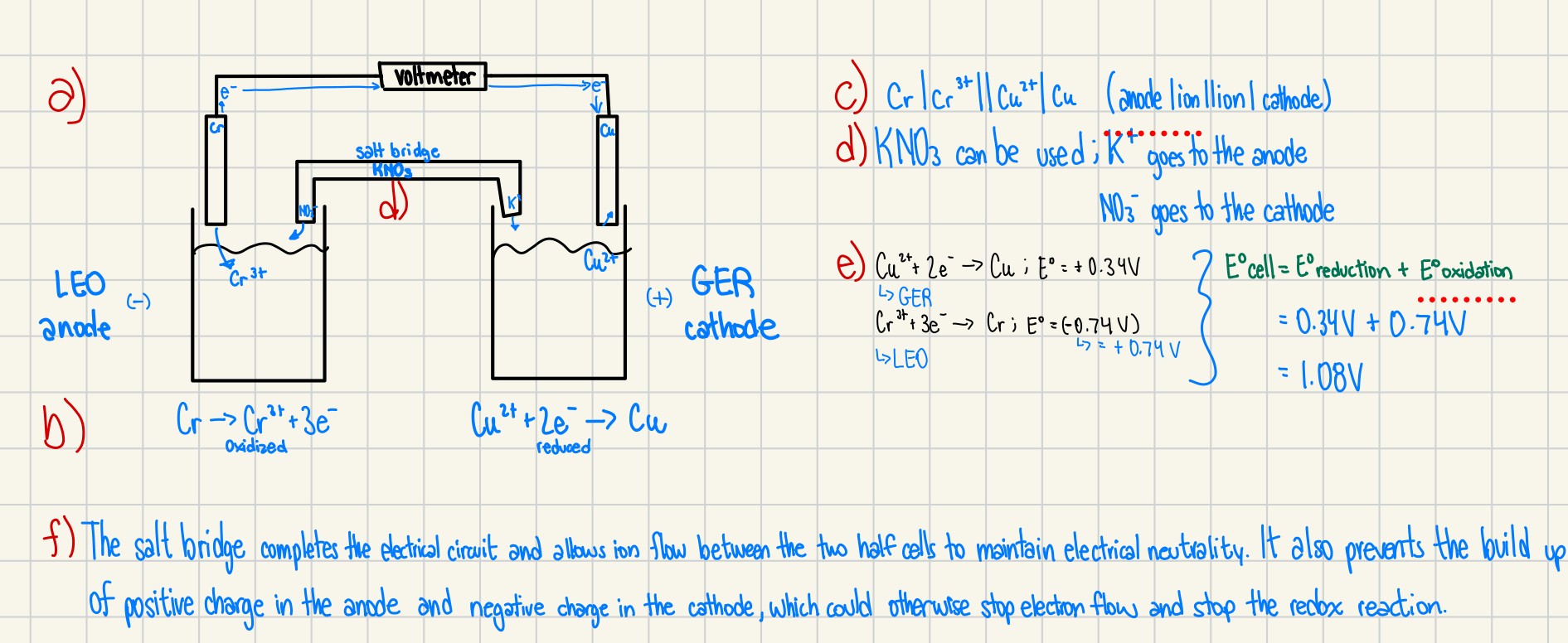
4. A functioning galvanic cell is produced using an aluminum electrode in an aluminum sulfate solution and a nickel electrode in a nickel(II) sulfate solution.
Cu²⁺ + 2e-⁻ → Cu E° = (+0.34 V)
Cr³⁺ + 3e⁻- → Cr E° = (–0.74 V)
(a) Sketch the galvanic cell and label the anode and cathode, ion movement in the electrolyte, and the direction of electron flow in the external circuit. (4 marks)
(b) Write the two half-reactions below their appropriate half-cell and identify them as oxidation or reduction. (2 marks)
(c) Give the line notation for the cell. (1 mark)
(d) Propose a material that could be used in the salt bridge and show the ion movement. (1 mark)
(e) What voltage would a voltmeter read? Show the work. (3 marks)
(f) Explain the purpose of the salt bridge in a galvanic cell. (2 marks)
f) The salt bridge completes the electrical circuit and allows ion flow between the two half-cells to maintain electrical neutrality.
It prevents the buildup of positive charge in the anode and negative charge in the cathode, which would otherwise stop electron flow and halt the redox reaction.
6. Use the table of standard reduction potentials to determine if the following reactions would be spontaneous or non-spontaneous as written. Explain how you arrived at your decisions. (4 marks)
a) 3Zn²⁺(aq) + 2Cr(s) → 2Cr³⁺(aq) + 3Zn(s)
b) 2Al(s) + 3Fe³⁺(aq) → 3Fe(s) + 2Al³⁺(aq)
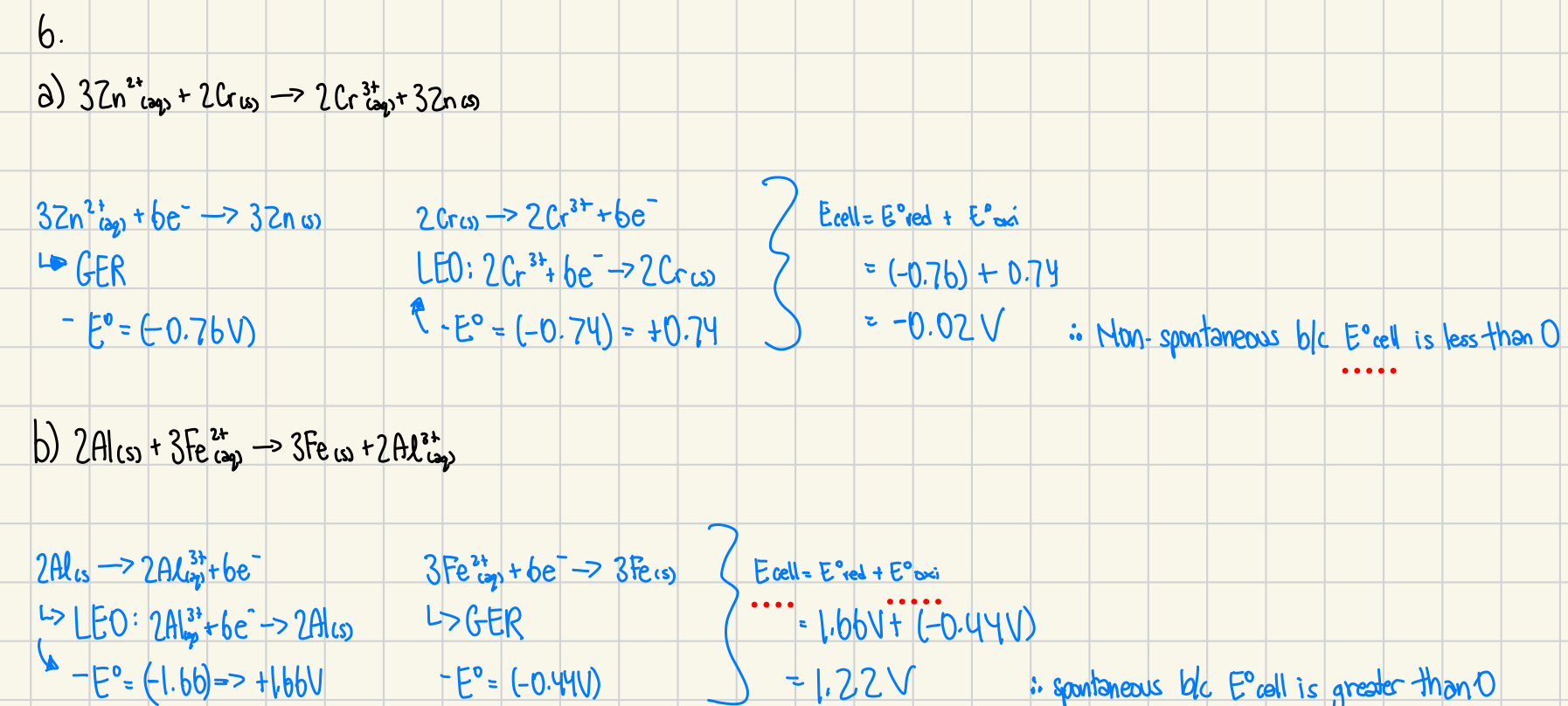
7. Suppose you have a galvanic cell in which the electrodes are zinc and iron. Write the redox reaction for the spontaneous galvanic cell and calculate the standard cell potential. (3 marks)

8. There are many practical applications of electrochemistry. In terms of electrochemistry, explain ONE of
i. a dry cell battery
ii. sacrificial anode or
iii. electroplating or
iv. galvanic corrosion (such as the 'lasagna cell') (3 marks)
i. There are two common types of dry cells: acidic and alkaline. Both types use two electrodes and an electrolyte paste to create a flow of electrons. In acidic dry cells, the paste contains MnO₂ and an acidic electrolyte, and the electrodes are separated by a piece of paper. For the cell to work, the anode and cathode must be connected so electrons can flow.
In alkaline dry cells, the paste uses a basic electrolyte like KOH instead. The zinc casing acts as the anode (where oxidation happens), while a carbon rod or brass rod serves as the cathode (where reduction happens). These batteries are used in many everyday devices because they are portable and reliable.
ii. A sacrificial anode is a metal (usually zinc or magnesium) that is more reactive than the metal it protects (like iron or steel).
In electrochemical terms, the sacrificial metal acts as the anode and undergoes oxidation, losing electrons and corroding instead of the protected metal.
This prevents rust or corrosion on important structures like ship hulls or pipelines by directing oxidation to the sacrificial metal.
iii. Electroplating uses a non-spontaneous redox reaction driven by an external power source (like a battery) to coat a metal object with a thin layer of another metal.
The object to be coated is placed at the cathode (where reduction happens), and the metal used for coating is at the anode (where it dissolves into ions).
This process improves appearance, prevents rust, and can reduce wear (e.g., silver-plating cutlery).
iv. Galvanic corrosion happens when two different metals are in contact in the presence of an electrolyte, forming a spontaneous electrochemical cell.
The more reactive metal becomes the anode, where oxidation occurs, and it corrodes over time. The less reactive metal becomes the cathode and is protected.
A real-life example is the “lasagna cell,” where aluminum foil (anode) touches a steel tray (cathode) with acidic tomato sauce acting as the electrolyte. This creates a mini galvanic cell, causing the aluminum to corrode and pit.
This process can also happen on ships, cars, or even dental fillings if dissimilar metals are exposed to moisture or electrolytes.
Bonus: Balance the following reaction
OCl- → ClO3- + Cl-
(3 marks)
Balance the redox reaction (acidic solution):
OCl⁻ → ClO₃⁻ + Cl⁻
Step 1: Split into half-reactions
Oxidation:
OCl⁻ → ClO₃⁻
Reduction:
OCl⁻ → Cl⁻
Step 2: Balance each half-reaction
Oxidation Half-Reaction:
Balance O by adding 2 H₂O to the left:
OCl⁻ + 2H₂O → ClO₃⁻Balance H by adding 4 H⁺ to the right:
OCl⁻ + 2H₂O → ClO₃⁻ + 4H⁺Balance charge by adding 4 e⁻ to the right:
OCl⁻ + 2H₂O → ClO₃⁻ + 4H⁺ + 4e⁻
Reduction Half-Reaction:
Add H₂O to the right (balance O):
OCl⁻ → Cl⁻ + H₂OAdd 2 H⁺ to the left (balance H):
OCl⁻ + 2H⁺ → Cl⁻ + H₂OAdd 2 e⁻ to the left (balance charge):
OCl⁻ + 2H⁺ + 2e⁻ → Cl⁻ + H₂O
Step 3: Multiply to equalize electrons
Oxidation (×1):
OCl⁻ + 2H₂O → ClO₃⁻ + 4H⁺ + 4e⁻
Reduction (×2):
2OCl⁻ + 4H⁺ + 4e⁻ → 2Cl⁻ + 2H₂O
Step 4: Add and simplify
Add both sides:
3OCl⁻ + 2H₂O + 4H⁺ → ClO₃⁻ + 2Cl⁻ + 2H₂O + 4H⁺
Cancel 2H₂O and 4H⁺ from both sides:
Final Answer:
3OCl⁻ → ClO₃⁻ + 2Cl⁻