Ap chem
1/13
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
What are the (Arrhenius) strong acids?
completely disassociate in water + increase h+ concentration:
HCl (Hydrochloric acid)
HNO3 (Nitric acid)
H2SO4 (sulfuric acid)
HBr (hydroponic acid)
HI (Hydroiodic acid)
HClO4 (Perchloric acid)
HClO3 (chloric acid)
What are the (Arrhenius) strong bases
A compound that completely ionizes in water + increase OH- concentration:
Potassium hydroxide (KOH)
Sodium hydroxide (NaOH)
Barium hydroxide (Ba(OH)2)
Caesium hydroxide (CsOH)
Strontium hydroxide (Sr(OH)2)
Calcium hydroxide (Ca(OH)2)
Lithium hydroxide (LiOH)
Rubidium hydroxide (RbOH)
How are Bronsted-Lowry acids/bases different from Arrhenius acids/bases?
Bronsted-Lowry concept extends to any molecule (not just OH- and H+) or substance, where an (Bronsted-Lowry) acid is a proton donor and a base is a proton acceptor.
How is a Lewis acid/base different from an Arrhenius acid/base?
Lewis acids/bases revolve around electrons, instead of protons.
-Lewis acids: receive electrons (yes receive)
-Lewis bases: donate electrons
Amphiprotic
A substance capable of acting as either an acid or a base
Ex. H2O can turn into OH- or H3O+
Conjugate base
A base formed by removing the proton from an acid
OH- is the conjugate base of H2O
Conjugate Acid
Acid formed by adding a proton to the base
H3O+ is the conjugate acid of H2O
Conjugate Acid-base pair
When an acid donates a proton, it becomes a base. When a base receives a proton, it becomes an acid.
Polyprotic acids/bases
Acids that are capable of losing more than a single proton in an acid-base reaction/Acids that have more than one ionizable H+ atom.
Ex. H2SO4
Bases that can receive more than one proton in a acid-base reaction
Ex. PO4³-
Acid dissociation constant + base dissociation constant
Ka; greater Ka =stronger acid.
Kb, larger = stronger base:
When will [H] = [OH]
At the equivalent point
What is a buffer?
A solution with a stable pH; adding an acid or a base doesn’t greatly affect the pH (Unless it is a big amount). Formed by adding a weak acid/base and its conjugate:
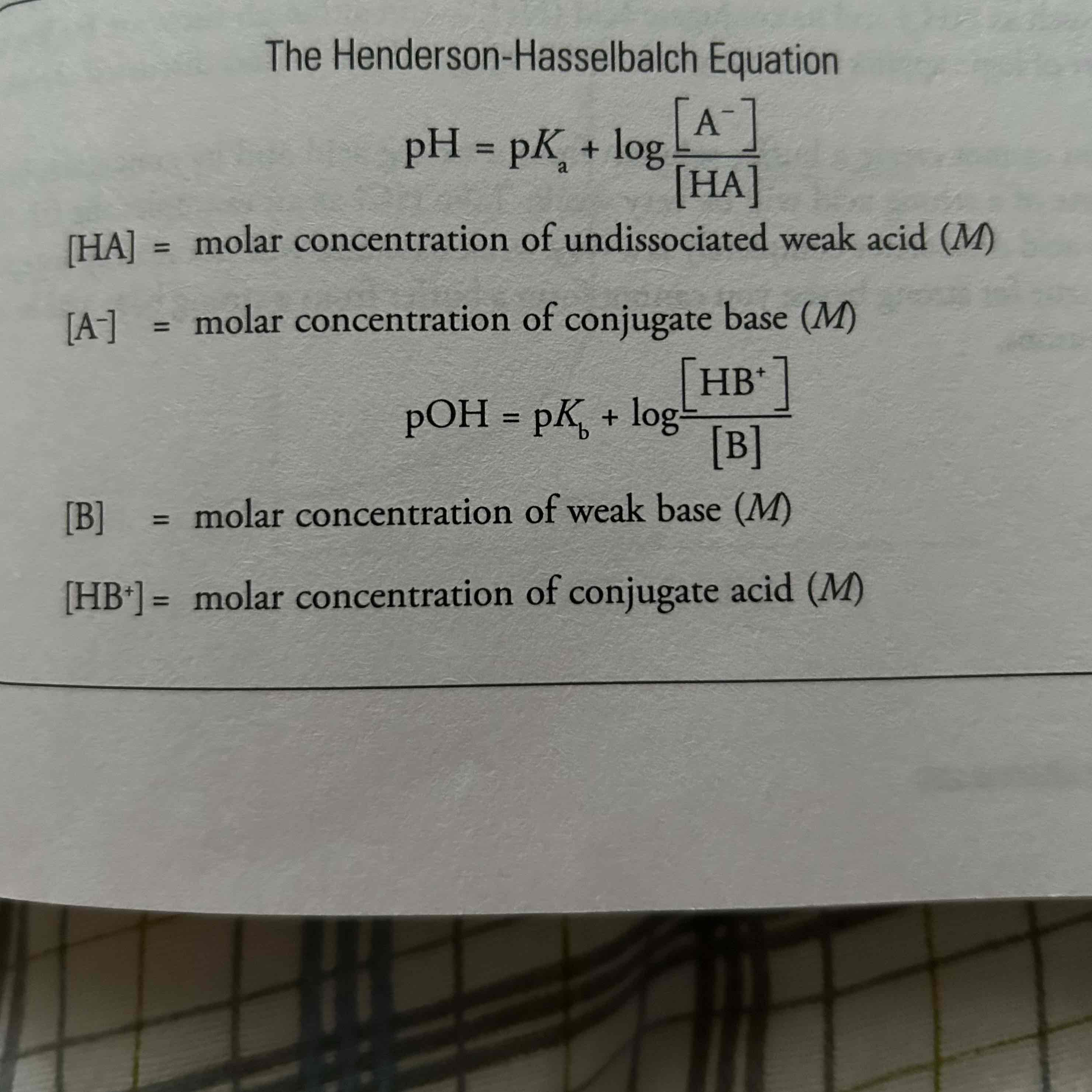
How do we calculate pH of a buffer?
The Henderson-Hasselbalch Equation