11CHEM Kinetic Theory and Reaction Rate
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
45 Terms
What is the kinetic theory of ideal gases
. a theory that explains the macroscopic properties of gases, such as pressure, volume, and temperature, by examining the random motion of gas molecules
. helps to explain the behaviour of gases under different conditions
. makes a series of general assumptions about all gases to make predicting their behaviour easier
. allows people to calculate the properties of a sample of gas such as pressure + temp when presented with info about that sample
What does the kinetic theory of ideal gases propose
Gases consist of molecules (except noble gases which consist of atoms) that move in continual random straight line motion
The average distance between gas molecules is very large compared to the size of the molecule
IMFs between molecules are negligible
All collisions of gas molecules are perfectly elastic collisions, meaning there’s no net energy loss during these collisions
Pressure is due to collisions of the molecules with the walls of the container
Temperature is a measure of the average kinetic energy of the molecules
Describe when the 6 kinetic theory of gases assumptions no longer work
. the ideal gas law fails at low temp and high pressure because the volume occupied by the gas is quite small, so the inter-molecular distance between the molecules decreases
—> hence an attractive force can be observed between them
Temperature of a substance
. a measure of the average kinetic energy of the particles
Pressure of a gas
. a measure of the number of collisions of the gas particles with each other and with the sides of the container that they’re in
Describe the 5 physical properties of gases
Gases diffuse: because gas particles have rapid, random motion and a lot of empty space between them so they quickly spread + mix
Gases can be easily compressed: because there’s a lot of empty space between particles
Gases spread to fill a container: because there are negligible forces between particles
Gases exert pressure: because the particles move rapidly + collide with the surface of any container or object
Gases have low density: because there’s a lot of empty space between them
Effect of volume on gas pressure
. decrease in the volume of a container, the gas pressure increases, and if you increase the volume, the pressure decreases
—> because a smaller volume leads to more frequent collisions of gas particles with the container wall
—> Boyle’s law: At a constant temperature, gas volume and pressure have an inverse relationship
Effect of temperature on gas pressure
. Increasing the temperature of a gas increases its pressure
—> because the gas molecules gain kinetic energy, move faster, and collide more forcefully and frequently with the container walls
—> Gay-Lussac's Law: states that pressure is directly proportional to absolute temperature (in Kelvin) when the volume and amount of gas are kept constant.
Describe the relationship between temperature and the kinetic theory of gases
. kinetic theory states that temp is a measure of average kinetic energy
. at a given temp, molecules of all gases, have the same average kinetic energy
. Kinetic energy is determined by mass and speed
—> heavier gases will have lower speeds than lighter gases at the same temp
. as the temp increases, the kinetic energy of the particles increases, and vice versa
. gas particles have a range of speeds at any given temp (the average speed increases as temp increases)
What is the absolute (kelvin) temperature
. a measure of the average kinetic energy of a gas’ molecules
. begins at absolute zero (0k), the point where molecular motion theoretically ceases
. This scale is used in gas laws like Charles's Law, which states that the volume of a gas is directly proportional to its absolute temperature when pressure is held constant
Describe what gas pressure is
. gas particles are far apart, in constant random motion, collide and exert pressure
. gas pressure = the force exerted by gas molecules colliding with the surfaces of their container, and it is calculated as force per unit area
. SI unit of gas pressure: the pascal (Pa) and is equivalent to a force of 1 newton per square metre
. the pressure due to the atmosphere = atmospheric pressure
—> standard atmospheric pressure at sea level = 1.00 × 10^5 Pa (100kPa)
What are the 2 standard conditions for gases
. standard conditions are used to compare changes in conditions that involve gases:
STP at 0°C and 100kPa (standard temp and pressure)
SLC at 25°C and 100kPa (standard laboratory conditions)
Describe Boyle’s law and its rule
. at a constant temp, the volume of a given mass of gas is inversely proportional to the pressure
—> larger the pressure, smaller the volume
. P1V1=P2V2
—> the units for P1,P2 (pressure) and V1,V2 (volume) must be consistent
What is Charle’s law
. at constant pressure, the volume of a fixed quantity of gas is proportional to its absolute temp
—> increasing gas’s temp increases the KE + speed of its particles, causing them to move faster + spread further apart —> to maintain a constant pressure, the gas must expand, increasing its volume to allow for more space between the rapidly moving particles
How can Charle’s law be combined with Boyle’s law
. can combine charle’s + boyle’s laws, because temp and pressure usually change together
—> gives a relationship between volume, pressure and absolute temp for a given mass of gas
. (P1V1/T1) = (P2V2/T2)
What is Avogadro’s Hypothesis
. states that equal volumes of any gas, measured at the same temp + pressure, contain the same number of particles
What is the molar volume of a gas (at STP) and in general
. 22.71L/mol at standard temp and pressure (0°C and 100kPa)
. is the volume occupied by 1 mole of any gas under specific temp + pressure conditions
What does the ideal gas law/general gas equation show
. shows the relationship between pressure, temp, volume, and number of moles of a gas
Rate
. how quickly one quantity changes compared to another quantity
Surface area
. the total area of all surfaces
Reaction rate
. refers to the speed at which reactants are consumed, or products are formed over a period of time
. the speed at which reactants are transformed into products, measured as the change in concentration of a reactant or product over a specific time interval
. the change in one measurable quantity over the change in time
—> measurable quantities for reactions (must be a quantifiable variable): mass, volume, pH, concentration, temperature
How is reaction rate found from values on a graph and calculated in general
. calculating the gradient (rise/run)
. rate of reaction = (amount substance used or produced)/(time taken)
Reaction rate graphs
. shows the changes during a reaction
. the gradient represents the rate of a reaction showing how quickly the reactants or products change
What is the collision theory
. a model that allows people to explain what happens during a chemical reaction, as well as the factors affecting it
Collision theory definition
. a successful collision (where chemical reaction occurs) requires the reactant particles to collide with sufficient activation energy and in a correct orientation
—> rate of the reaction is determined by the frequency of these "successful" collisions.
Why does reaction rate generally slow as a reaction progresses
. as reaction goes on, concentration of reactants decreases, meaning reactant particles are further apart, leading to less collisions, and a slower reaction rate
Activated complex/transition state
. a very short-lived, high-energy, and unstable arrangement of atoms that forms at the peak of the energy barrier during a chemical reaction
—> represents the point where bonds are in the process of breaking and forming, and it must be reached for reactants to convert into products
—> The energy required to reach this state is called the activation energy, and it dictates the rate of the reaction.
. the brief moment when the bonds of the reactants have broken, but the bonds of the product haven’t formed, as the reactants are forming products
Describe the energy of the activated complex relative to reactants and products
. as energy was required to break the bonds of reactants, the activated complex has more energy than the reactants or products
—> as the activated complex forms new bonds to produce the products, energy is released
Enthalpy
. the total energy possessed by a substance
. the reactant, activated complex and product all have different enthalpies
Activation energy
. the amount of energy needed to form the activated complex
. as this energy is needed for bonds of reactants to break, the activation energy is the min amount of energy required to initiate a chemical reaction determined from the difference in energy between the activated complex and reactants
Describe how the Maxwell-Boltzmann Distribution relates to successful collisions
. at a given temp, particles will have different amounts of kinetic energy, with the average kinetic energy determining the temp
. only particles with sufficient or greater energy to break the bonds of the reactant particles are able to have a successful collision
Identify the main factors affecting reaction rate (4)
Concentration
Surface area
Temperature
Catalysts
. agitation (stirring increases as more particle collisions)
Factors affecting reaction rate: concentration
. concentration refers to the number of particles in a given volume (the amount of solute dissolved in a given volume of solvent)
. pressure is determined by concentration of gas particles
. Increased concentration = higher amount of dissolved reactants per volume unit
—> increases the number of successful collisions because the particles are closer together = more collisions = faster rate of reaction
Factors affecting reaction rate: Surface area
. when a particle exists as a solid, only the particles on the surface are available for reacting
—> once surface particles have reacted + moved away, other particles below are available for reacting
. if a solid is divided or crushed, more particles are exposed as surface particles = more collisions can happen at once = faster rate of reaction
Factors affecting reaction rate: Temperature
. temp is a measure of average kinetic energy
. increased temp = increased percentage of successful collisions because:
—> more particles have sufficient activation energy to form the activated complex
—> increased number of collisions because the particles now have greater speed + can collide more frequently
. maxwell-boltzmann distribution is pushed to the right
. reactant particles are higher energy + move faster leading to more collisions with sufficient activation energy
Catalyst
. a substance that affects the rate of reaction without being consumed in the reaction (chemically unchanged at end of reaction)
. can be positive or negative
—> often speed up a chemical reaction
—> when reaction has finished, catalyst mass is unchanged + can retrieve for further use
Factors affecting reaction rate: Catalysts
. provide an alternative reaction pathway for the reaction to occur, which has a lower activation energy = more particles with sufficient energy to successfully collide = more successful collisions = faster reaction rate
—> form a different activated complex (reaction occurs via a different pathway), not just lower the energy required for same pathway (original activation energy stays the same)
. catalysts also orient reactants correctly, increasing effective collisions
Examples of catalyst (2)
Metal nanoparticles/nanomaterials:
. a particle with a diameter of less than 100nm that behaves as a whole unit
. inorganic catalysts
—> advantage: large surface area (lots of surface particles for reaction to take place)
Enzymes:
. proteins that ensure reactions important for life occur at an appropriate rate
. organic catalysts
. affected by temp and pH
—> disadvantage: made of protein which can denature (changes shape so will lose function as enzymes function due to their shape)
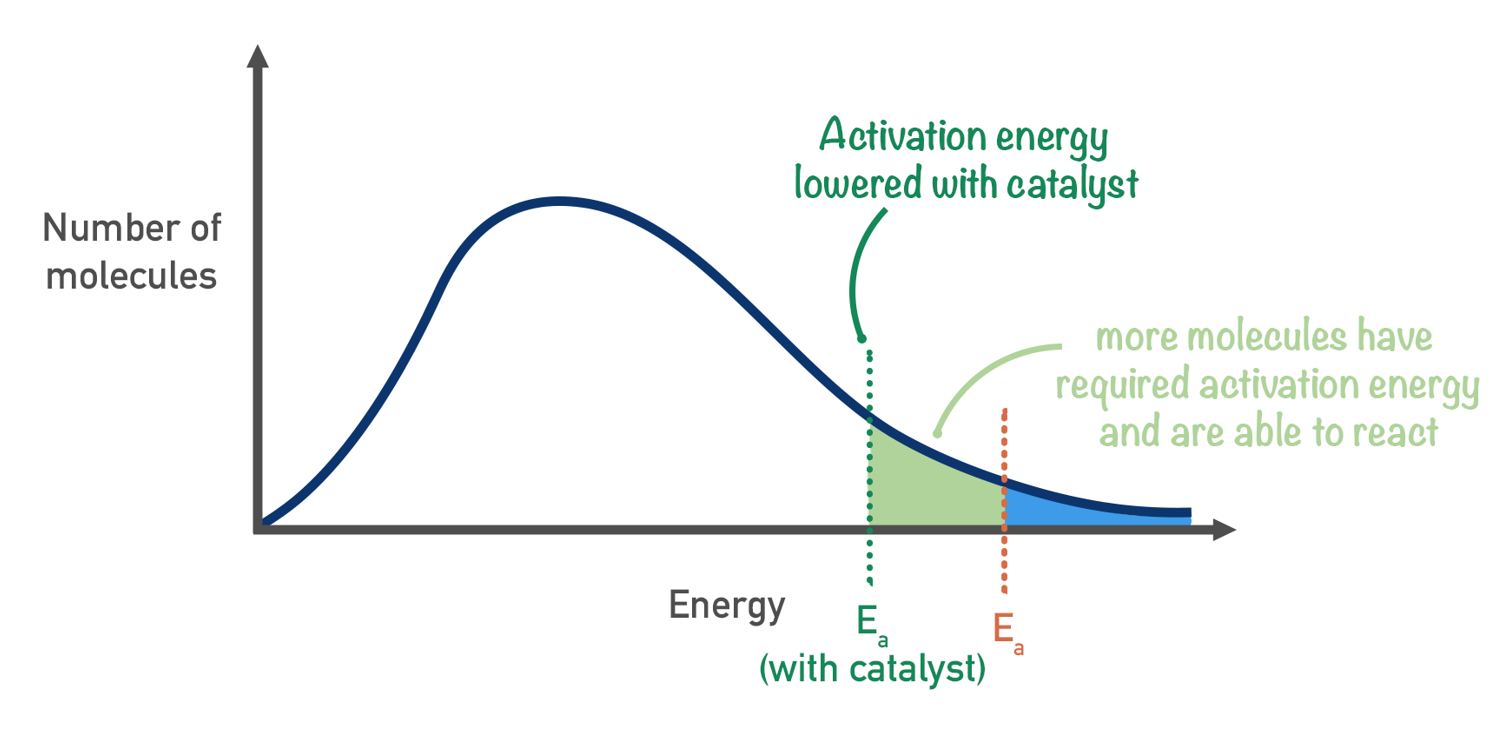
Catalyst affect on Maxwell-Boltzmann Distribution diagram
. don’t change the shape of the distribution
. provide a new pathway with lower activation energy
. more particles have enough energy to successfully react
. catalysts also orient reactants correctly, increasing effective collisions
Surface (heterogenous) catalysts
. a substance, often a solid, that increases the rate of a chemical reaction by providing a surface for the reactants to interact
—> different state from reactants (usually solid catalyst, gas/liquid reactants —> often used to speed up reactions of gases)
—> surface holds + orients molecules correctly
—> catalyst’s surface provides active sites where reactant molecules adsorb, and bonds break/form, therefore undergoing the reaction faster
—> EG: iron in Haber process (N2+H2—>NH3), platinum in catalytic converters
Homogenous catalysts
. substances that speed up a chemical reaction by being in the same phase as the reactants
—> catalyst in the same state as reactants (often liquid/aqueous)
—> there is a high degree of interaction between the catalyst and the reactant molecules because they are all mixed together (since the catalyst is uniformly distributed throughout the reaction mixture, it can interact closely with the reactants, allowing for very selective and precise reactions)
—> forms temporary intermediates (molecule made between the reactants and products)
—> provides a new reaction pathway with lower activation energy
—> harder to separate
—> common in biological + solution-based systems
—> most commonly a dissolved catalyst in a solution or a gas-phase catalyst with gas-phase reactants
—> EG: using a dissolved iron ion to catalyse a reaction between other aqueous ions
Catalytic converters
. a device in a vehicle's exhaust system (of petrol cars) that converts harmful pollutants (toxic gases) from engine exhaust into less harmful gases, such as water vapor and carbon dioxide, through chemical reactions
—> use heterogenous catalysts (solid metal catalysts like Pt, Pd, Rh, with gas reactants)
—> reaction occurs on the surface of the metal
—> they don’t increase the energy content of the fuel being combusted
Key reactions for catalytic converters (3)
carbon monoxide (CO) —> carbon dioxide (CO2)
Nitrogen oxides (NOx) —> nitrogen gas (N2)
Unburnt hydrocarbons (HC) —> carbon dioxide + water
Random errors and what they affect
. an error in measurement caused by factors which vary from one measurement to another (inconsistencies)
—> each trial affected differently
—> affect (reduce) reliability
—> EG: parallax meniscus reading error, inconsistent stopwatch timing, slight temp variations of lab during repeated trials
Systematic errors and what they affect
. a consistent and repeatable error that causes measurements to deviate from the true value in the same direction and by the same amount
—> affect results in same way each trial
—> affect (reduce) validity
—> EG: balance improperly scaled, contaminated reagent used in all trials, miscalibration of equipment