ACS FINAL EXAM
1/158
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
159 Terms
According to the Bronsted-Lowery definition which chemical species can function both as an acid and as a base?
HCO3-
In the reaction CN-+H2O ←→HCN +OH- which is an acid -base conjugate pair?
Given that HX is a stronger Bronsted acid than HY in aqueous solution, which is true of 1M solution of NaX
the pH of A 0.03M HCL solution is
The pH of a 1.0 × 10-3M Ba(OH)2 solution at
Which is the strongest acid?
Which salt reacts with water (hydrolyzes) to produce a basic solution?
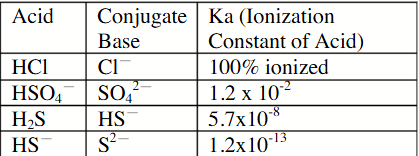
The weakest of the base is
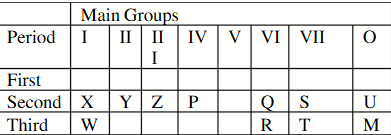
The oxide of which element will react with water to form the strongest acid?
Which statement is a logical inference from the fact that 0.10 M solution of potassium acetate KC2H3O2, is less alkaline than a 0.10 M solution of potassium cyanide, KCN?
In the titration of 50.0 mL of 0.100 M benzoic acid (a monoprotic acid) with 50.0 mL of 0.100 M NaOH, the properties of the solution at the equivalence point will correspond exactly to the properties of
A mixture of which pair of 0.1 M aqueous solutions would constitute a buffer?
what do these have in common? 20Ne 19F1- 24Mg2+
The number of neutrons in the nucleus of an atom of 1327Al is
The orbitals of 2p electrons are often represented as being
The element in period 5, Group 3A, has the outer electron configuration
Which electron configuration is impossible?
The element X occurs naturally to the extent of 20.0% 12X and 80.0% 13X. The atomic mass of X is nearest
Which electron transition is associated with the largest emission of energy?
If an electron moves from one energy level in an atom to another energy level more remote from the nucleus of the same atom
A photon light of 450 nm, when compared to light of wavelength 300 nm, has (1nm=10-9 m)
which set of quantum numbers is possible for an electron in an atom?
A compound consisting of an element having a low ionization potential and a second element having a high electron affinity is likely to have
according to modern bonding theory the number of sigma and pi bonds in the ethylene molecule H2C=CH2 is
The number of sigma bons i N=N is
The elements in an ionic compound are held together by
In every electrolytic and galvanic (voltaic) cell the anode is that electrode
Metal X was plated from a solution containing cations of X. The passage of 48.25 C deposited 31 mg of X on the cathode. What is the mass of X (in grams) per mole of electrons?
In a galvanic (Voltaic) cell in which the reaction is Cd+Cu2+→Cu +Cd2+ and the ions are at unit concentration (activity), the cell potential is
Cd→Cd2++2e- 0.4021 V
Cu→Cu2++2e- -0.344 v
In which reaction will an increase in total pressure at constant temperature favor formation of the products?
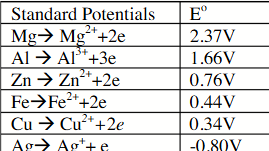
using only metals Mg, AL, Zn, Fe, Cu and Ag, together with their 1 M salt solutions, a voltaic cell of the highest possible voltage would be constructed using electrodes of these metals.
E=E0 -0.059/n log Q (Nernst equation)
[H+] =1.0 M initially + P02 = 1. atm
4e +O2(g)+4H+(aq)←→2H2O(l) E=1.23 V
based on the information above, which statement is correct?
The equilibrium constant for the gaseous reaction C+D←→ E + 2F is 3.0 at 50 C. In a 2.0L flask at 50 C are placed 1.0 mol of C, 1.0 mol of D, 1.0 mol of E, and 3.0 mol of F. Initially, the reaction will
At 298 K the equilibrium constant for H2(g)+1/2 O2(g)←→H2O(l)
Consider the reversible system at equilibrium:
2CO+O2←→2CO2 + heat
when the temperature is increase at constant pressure
The numerical value of the equilibrium constant for any chemical change is affected by changing
What is the equilibrium constant expression for the gas phase oxidation of CO to CO2 by O2?
Into an empty vessel COCl2(g) is introduced at 1.0 atm pressure whereupon it dissociates until equilibrium is established 2COCL2(g) ←→ C (graphite) + CO2(g) +2CL2(G)
At a certain temperature, the equilibrium constant for the reaction 2HI(g) ←→H2(g) + I2(g) is 0.49. Calculate the number of moles of hydrogen produced when one mole of HI is placed in a 1 L vessel at this temperature.
What us the [OH] of a solution which is 0.18 M in ammonium ion AND 0.10 m In ammonia? Kb=1.8 × 105
What is the pH of a 0.10 M solutions of a monoprotic acid, HA, with a Ka = 1.0 × 106
When 0.10 mol of a weak acid HA was diluted to one liter, experiment showed the acid to be 1% dissociated.
HA+H2O←→ H3O+ + A
What is the acid dissociation constant, Ka?
Which solution has a p less than 7.0?
What is the pH of a 0.1 M NaF solution? Ionization constant for HF, Ka=7 × 104
What is the hydrogen ion concentration of a buffer solution containing 0.10 M NO2 and 0.20 M HNO2? Ionization constant for Nitrous Acid, Ka=4.5 × 10^4
The solubility of BaCO3 is 7.9 × 103 g/L. Calculate the solubility product, ksp ignoring hydrilysis. MW of BaCO3 197 g/mol
The addition of solid Na2SO4 to an aqueous solution in equilibrium with solid BaSO4 will cause
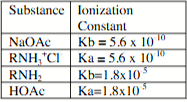
Assume the standardized aqueous solutions of each of these are available. A buffer with desired pH is 5.0 would be conventionally prepared by appropriate mixtures of
which substance is most soluble in water?
The solubility of BaCrO4 in water is 2.8 × 103 g/L what is the Ksp of the salt? MW of BaCrO4 253 g/mo
Which is the correct expression for the solubility product constant Ag2CrO4?
The correct IUPAC name of N2O3 is
In which case is the substance with the given formula followed by its correct name?
Balance the equation for the following reaction, using no fractional coefficients.
?C + ?HNO3→ ?CO2 +?NO2+?H2O
The sum of the coefficients in the balanced equation is
Complete and balance the equation for the reaction, where the reactants are in aqueous solution. Use no fractional coefficients.
?Na3PO4 + ?Ba(NO3)2→ +
The number of moles and formula of the product containing Ba are
According to the kinetic molecular theory
The volume of a given mass of gas varies inversely with pressure. Provided that the temperature remains constant because
The kelvin temperature of one liter of as is double and its pressure is tripled, volume will than be
Which gas, present in the same close system, has the greatest average kinetic energy at a given temperature?
A sam0le of neon occupies a volume of 27.3 L at STP. What would be the neon volume at 177 C and 0.100 atm pressure?
Under the same conditions of temperature and pressure, the gas whose molecules posses the highest average speed is
What is the colume of 2.00 mol of helium gas at 27 C and 3.00 atm
Reals gases are most like ideal gases at
500mL of a gaseous compund has a mass of 0.9825 g at 0 C and 760 mmHg. What is the approximate molar mass of the compund
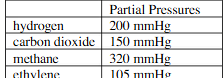
The partial of a gaseous mixture are given in table. what is the mole precent of hydrogen?
It is desired to collect enough oxygen over water at 25