SSC Exam 2
1/78
Earn XP
Description and Tags
These flashcards cover essential vocabulary and concepts related to soil science, including definitions and relationships important for understanding soil properties and nutrient management.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
79 Terms
Soil Colloids
Extremely small soil particles (0.1–0.001 µm) with large surface area and electrical charge that adsorb water and ions.
Types of Soil Colloids
Inorganic (clay minerals, Fe/Al oxides) and organic (humus).
1:1 vs 2:1 Clays
1:1 clays (kaolinite) one tetra to one octa, are older, non-expanding; 2:1 clays (montmorillonite) two tetra to one octa, are younger, shrink–swell.
Humus
Stable, dark organic matter with high cation exchange capacity (CEC) and pH buffering ability.
the more organic matter = darker soil color
has positive and negatic ends
Cation Exchange Capacity (CEC)
Sum of total cations that a soil can adsorb, expressed in meq/100g soil.
Isomorphic Substitution
process in which one element substitutes another of comparable size in the crystalline structure, creates a permanent charge.
pH-Dependent Charge
Charge arising from broken edges or organic matter; increases with pH.
Effect of pH on CEC
As pH increases, CEC increases because more negative sites are available for cation adsorption.
Cation Adsorption Strength Order
Al3+ > Ca2+ > Mg2+ > K+ = NH4+ > Na+ > H+.
Soil Acidity
Soils with pH below 7.0; each 1 unit drop equals 10× more acidity.
Active Acidity
H+ and Al3+ ions in soil solution; measured by pH meter.
Exchangeable Acidity
H+ and Al3+ held on cation exchange sites of clays and organic matter negative sites.
Residual Acidity
H+ and Al3+ bound to soil particles and not exchangeable.
Buffering Capacity
Soil’s ability to resist changes in pH; increases with clay and organic matter content.
Liming
Process of adding calcium or magnesium carbonates to raise soil pH and reduce acidity.
Calcitic vs Dolomitic Lime
Calcitic lime = CaCO3; Dolomitic lime = CaMg(CO3)2 (adds Mg).
Fineness of Lime
Smaller particle size = faster reaction and greater surface area.
Essential Plant Nutrients
17 total; 14 from soil (N, P, K, Ca, Mg, S, Fe, Mn, B, Zn, Cu, Cl, Mo, Ni).
Liebig’s Law of the Minimum
Plant growth is limited by the most deficient nutrient, even if others are adequate.
Macronutrients
N, P, K (primary); Ca, Mg, S (secondary).
Micronutrients
Fe, Mn, B, Zn, Cu, Cl, Mo, Ni – required in trace amounts.
Nutrient Movement to Roots
Mass flow, diffusion, and root interception.
Mycorrhizae
Fungal associations that increase root surface area and nutrient uptake.
Nitrogen Forms
Nitrate (NO3–) and ammonium (NH4+).
Symbiotic Biological Nitrogen Fixation
Bacteria fix N2 to plant-available forms for plants to use. In return, bacteria get
sugars.
Microbes (Rhizobium) convert N2 gas to plant-available NH3 in legume root nodules.
Mineralization
Biological conversion of unavailable organic N into plant
available inorganic N.
Conversion of organic N to inorganic N (NH4+, NO3–) by microbes.
Immobilization
Conversion of inorganic N into organic forms within microbial or plant tissue.
N Loss Pathways
Leaching, denitrification, volatilization, and plant uptake.
Ammonification
First step of mineralization: organic N → NH4+.
Conversion of organic N to ammonium by microorganisms.
Need warm temperatures, good soil moisture, and oxygen supply.
Makes the soil more basic
Nitrification
NH4+ → NO2– → NO3– by Nitrosomonas and Nitrobacter; acidifies soil.
Conversion of ammonium to nitrite and then to nitrate.
Phosphorus Availability
Controlled by pH and clay; binds to Fe/Al at low pH and to Ca at high pH.
Phosphorus Environmental Impact
Excess P causes eutrophication and algal blooms.
Potassium Cycle
K+ is held on exchange sites or trapped in clay interlayers; no major environmental issues.
CEC and pH Relationship
Higher pH increases CEC due to deprotonation of negative sites.
Calcium and Magnesium
Supplied by calcitic/dolomitic lime; important for cell walls and chlorophyll.
Sulfur in Plants
Component of amino acids and proteins; deficiencies show as yellowing of new leaves.
Micronutrient Availability and pH
As soil pH increases, availability of Fe, Mn, Zn, and Cu decreases.
Copper and Zinc Toxicity
Occurs at low pH and with repeated manure application; managed by increasing pH or organic matter.
Soil Sampling Depth
Field crops: 8 in; perennials: 4 in.
Composite Sample
Combination of several subsamples representing one field or soil type.
Grid Sampling
Sampling on a regular grid to map soil variability and nutrient needs.
NCDA Soil Test Index
Unitless index value representing nutrient availability or heavy metal risk.
Realistic Yield Expectation (RYE)
Estimated yield for a soil-crop combination used to set N rate (N rate = RYE × N factor).
Four Rs of Nutrient Management
Right source, Right rate, Right time, Right place.
Cation Exchange and Flocculation
Ca2+, Mg2+, Al3+ promote flocculation; Na+ causes dispersion.
Source of Soil Acidity
CO2 reaction, organic matter decomposition, NH4+ oxidation, Al hydrolysis.
Role of Lime Reaction
Ca2+ replaces H+ and Al3+ on exchange sites; carbonate neutralizes acidity.
Plant Macronutrient Function – N
Promotes leaf growth, protein formation, and chlorophyll production.
Plant Macronutrient Function – P
Energy transfer and root development; deficiency causes purple leaves.
Plant Macronutrient Function – K
Water regulation and stress resistance; deficiency causes leaf scorching.
Soil pH Effects
Low pH increases metal toxicity; high pH decreases micronutrient availability.
CEC Units
Milliequivalents (meq) per 100 g dry soil.
Effect of Weathering
Older soils (Ultisols, Oxisols) are more acidic and low in nutrients.
Iron and Aluminum Oxides
Non-expansive, low CEC clays found in highly weathered soils.
Soil Buffering Mechanisms
Include mineral dissolution, Al compounds, cation exchange, and carbonates.
Organic Matter Benefits
Improves aggregation, water holding, aeration, and nutrient cycling.
pH Measurement Equation
pH = –log[H+]; lower pH = higher acidity.
Eutrophication
Nutrient enrichment (especially P) of water bodies leading to algal blooms and low O2.
CEC Influencers
Clay content, organic matter, and pH level determine soil’s cation exchange capacity.
Cation
Positive ion (+1)
Boron (depending on pH)
Calcium
Copper
Hydrogen
Magnesium
Manganese
Nickel
Nitrogen (Ammonium form, NH4+)
Potassium
Zinc
Anion
Negative Ion (-1)
Boron (depending on pH)
Chloride
Molybdate
Nitrogen (Nitrate form, NO3-)
Phosphate *
Sulfate
Clayey soil and soils with humus (organic matter) generally have ______ CEC.
higher
Highest —————————————————> Lowest
Buffering
Humus → 2:1 clays → 1:1 clays → oxides → sand
Beneficial effects of liming
Crop yield improvement
Nutrient availability effects
Improved microbial activity
Improved legume fixation of N
Ca & Mg addition:
Calcitic lime = only Ca
Dolomitic lime = Ca and Mg
Step 1 of Liming
Ca2+ from the lime replaces Al3+ and H+ on the cation exchange complex.
Step 2 of Liming
The carbonate reacts with the H+ ions, removing them from solution thereby raising the pH. Lime works by turning H+ to water.
Step 3 of Liming
Al3+ is hydrolysed to form Al-hydroxides and H+ ions. Then carbonate from the lime neutralizes the H+ generated during the Al-hydrolosis.
Calcium Carbonate Equivalent (CCE)
Neutralizing value of any liming material compared to pure calcium carbonate. (Higher CCE = Less lime)
Buffering capacity Higher buffering capacity = more lime
Cation exchange capacity CEC Higher CEC = more time
Lime type More pure lime = less lime
Lime fineness Smaller lime particle = changes pH faster
Soil texture More clay = More lime
Volatilization
Removal of N from the soil by turning it into a gas that leaves the soil.
Denitrification
Conversion of nitrate to a gas form of N (ideally N2).
Need warm temperatures, low oxygen, organic matter (carbon source).
Crop uptake and removal
Removal of N by plants and then removing plant residues from the field.
– Crop variety
– Soil type
– Climate
– N application (timing, source, placement, rate)
Plant Micronutrients
Iron
Manganese
Boron
Zinc
Copper
Chlorine
Molybdenum
Nickel
Non-essential but beneficial plant nutrients
Sodium
Silicon
Cobalt
Selenium
Aluminum
Mass Flow
Dissolved nutrients move to the root in soil water that is flowing towards the roots.
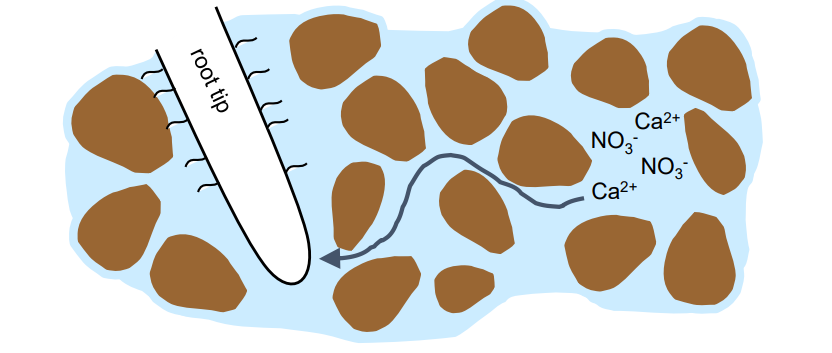
Diffusion
nutrients move from higher concentration in the bulk soil solution to lower concentration at the root
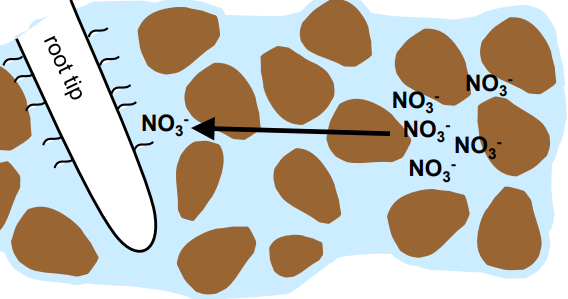
Root interception
roots obtain nutrients by physically contacting nutrients in soil solution or on soil surfaces.
A bag of 10-10-10 fertilizer is:
10% Plant-Available N
10% P2O5
10% K2O

A bag of 16-0-8 fertilizer is:
16% Plant-Available N
0% P2O5
8% K2O

Phosphorus Cycle
Available forms: H2PO4-, HPO42-
Strongly binds to Fe and Al oxides, so P stays where it is
placed in the soil.
P is most likely to be lost via erosion and moves very little down the soil profile.
Mediated by equilibrium chemistry