PCHEM EXAM 2
0.0(0)
Card Sorting
1/7
There's no tags or description
Looks like no tags are added yet.
Last updated 7:39 PM on 12/16/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
1
New cards
State the second law of thermodynamics
Any spontaneous process increases the entropy of the universe
2
New cards
State the third law of thermodynamics
Entropy of a perfectly crystalline substance at absolute zero temp (0K) is zero
3
New cards
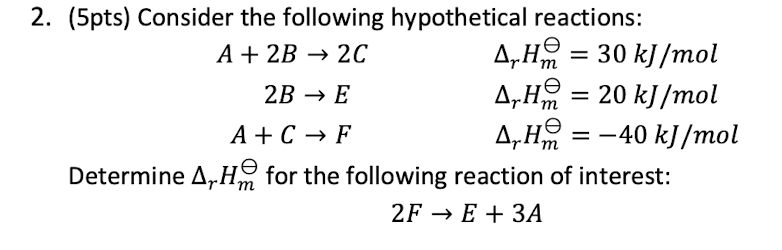
4
New cards

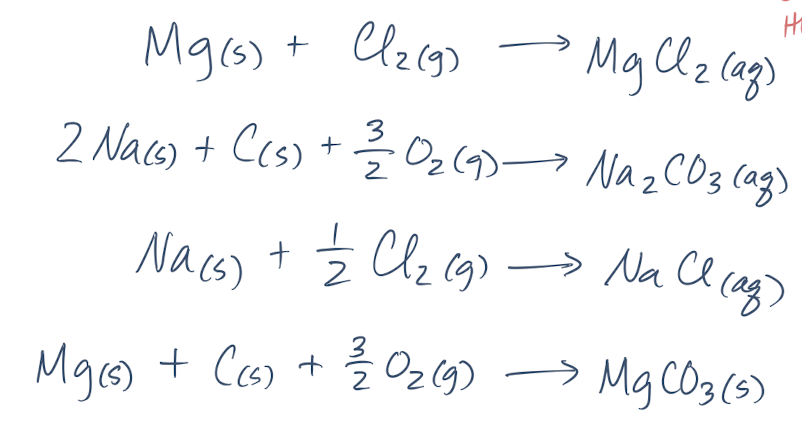
5
New cards

2.54 J/K
6
New cards

If the insulation was poor, then heat would escape the calorimeter. This would cause a smaller temperature change than expected, meaning |change in T| would be smaller than if the insulation was effective
7
New cards

For a bomb calorimeter, change in U=qu=C(changein)T. So, a smaller |change in T| will also cause a smaller measured q, and therefore a smaller |change in comb U|
8
New cards