Ch. 8 Alkene Reactions
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Hydrogenation - Catalytic Reduction
H2 / Pt —- Syn Addition
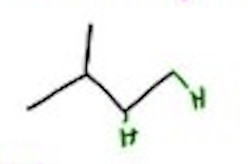
Hydrohalogenation
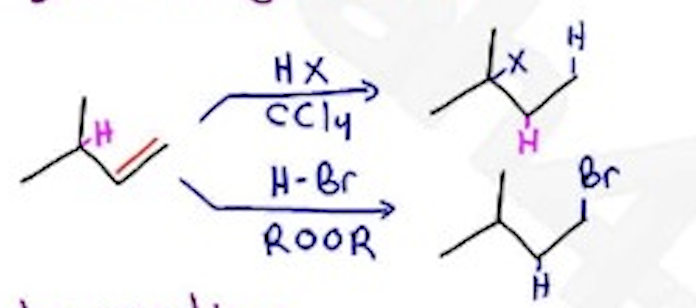
H-x / CCl4 —- Markovnikov, Hydrogen Shift, carbocation — X = Cl, Br, I
H-Br / ROOR —— ROOR = peroxides, Anti-markovnikov
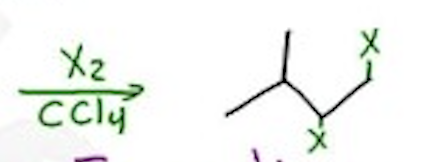
Halogenation
X2 / CCl4 —- Anti-addition —- X = Cl, Br
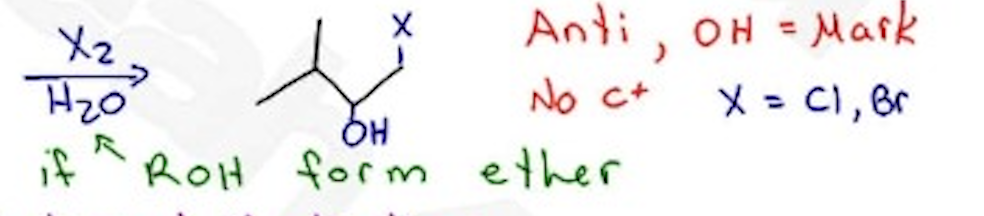
Halohydrin Formation
X2 / H2O —- Anti, OH = markovnikov, no carbocation, X = Cl, Br
X2 / ROH —→ Forms an ether
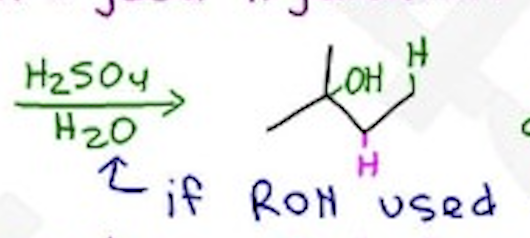
Acid Catalyzed Hydration
H2SO4 / H2O —- Markovnikov, H-shift, carbocation
If ROH is used, forms an ether
Oxymercuration - Reduction
Hg(OAc)2 H2O / NaBH4 —- Markovnikov, no H-shift, anti
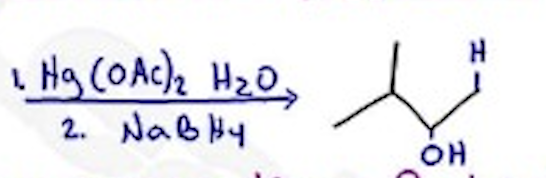
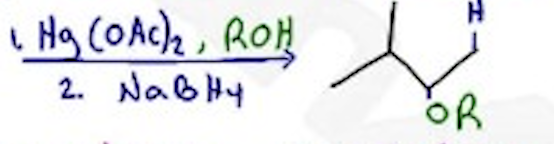
Alkoxymercuration - Reduction
Hg(OAc)2, ROH / NaBH4 —- Markovnikov, no H-shift, anti
Hydroboration - Oxidation
BH3, THF / NaOH, h2o2 —- Anti-mark, syn
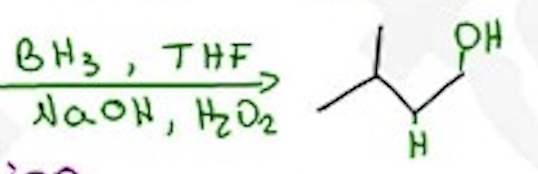
Epoxidation
Peracid —- syn (mCPBA is often used)
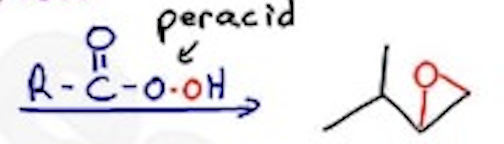
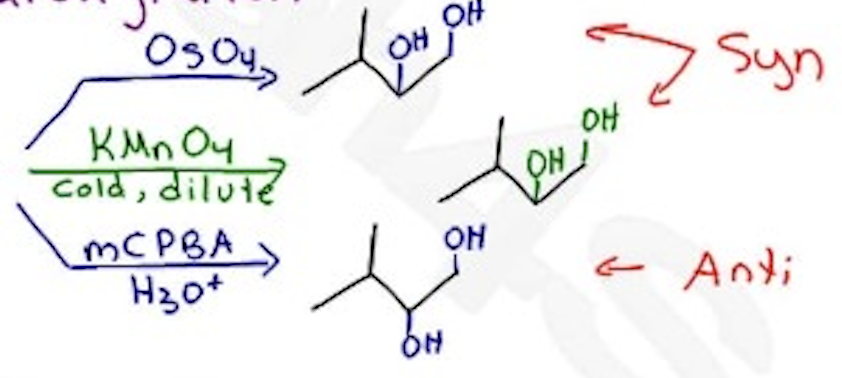
Dihydroxylation
OsO4 —-→ Syn
or KMnO4 —→ Syn
or mCPBA / H3O+ —→ Anti
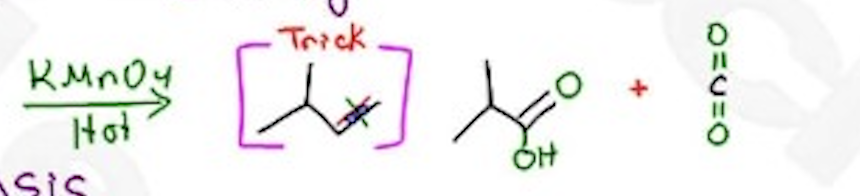
Oxidative cleavage
KMnO4 —>
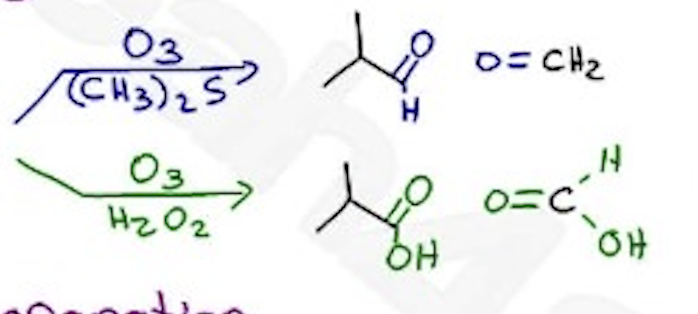
Ozonlysis
O3 / (CH3)2S —→ primary carbon aldehyde
O3 / h2o2 —→ primary carbon carboxylic acid
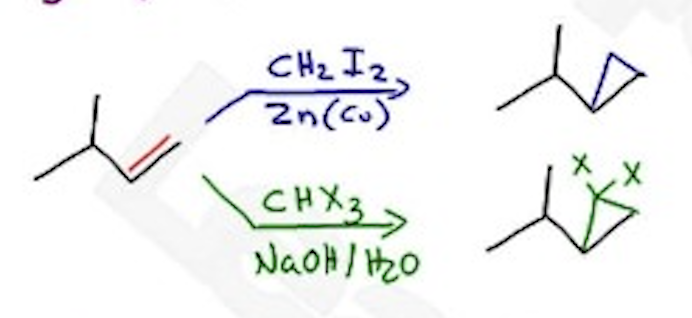
Cyclopropanation
CH2I2 / Zn(Cu) —→ Simmons-Smith Rxn
CH3X3 / NaOH / H2O —→ Simmons-Smith Rxn (with bonds from top of ring)
Syn
Meaning Syn Addition —> Both substituents are added to the same side of a double bond. BOTH WEDGE OR BOTH DASHES IN TERMS OF STEREOCHEMISTRY.
Mark
Meaning Markovnikov —> Something like Br will be added to the more substituted carbon (because it is more stable). The H atom will be added to a lower substituted carbon (like a primary carbon 1)
Anti
Meaning anti-addition. The added substituents are NOT on the same side. ONE IS DASHED AND ONE IS WEDGED. can be interchangeable**8
Anti-markovnikov
A substituent like Br will attach to a less substituted carbon. Like a primary carbon.