Oxidation of Alcohols
0.0(0)
Card Sorting
1/5
There's no tags or description
Looks like no tags are added yet.
Last updated 8:39 AM on 10/3/24
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
1
New cards
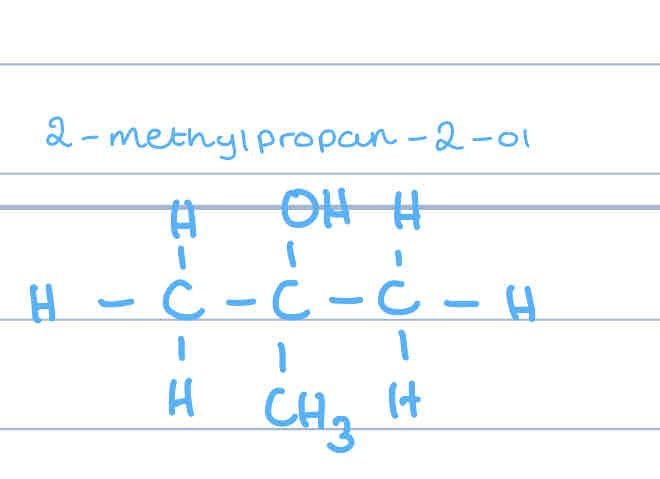
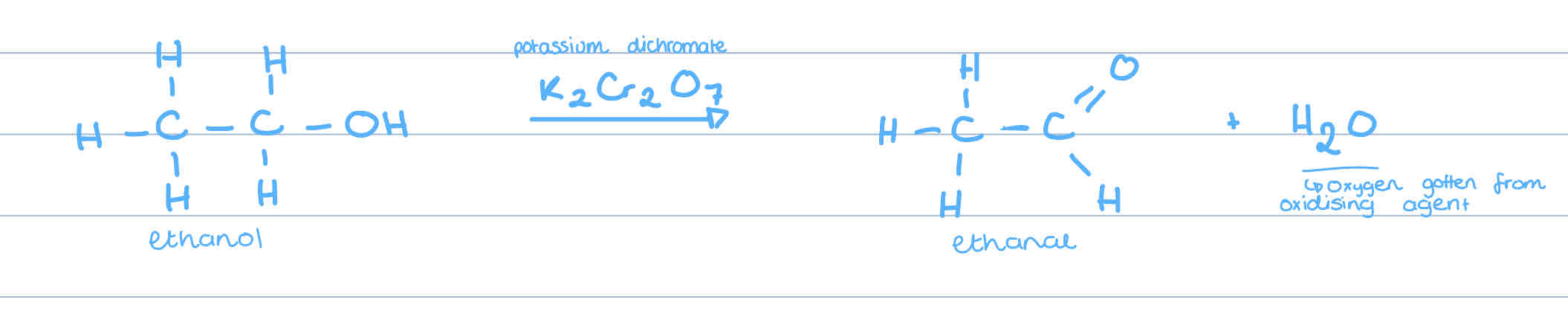
What do primary alcohols oxidise to?
aldehydes
2
New cards
Where do the molecules for H2O come from?
oxygen is gotten from the oxidising agent and the hydrogen comes from the carbon
3
New cards
What do aldehydes oxidise to?
carboxylic acid
4
New cards
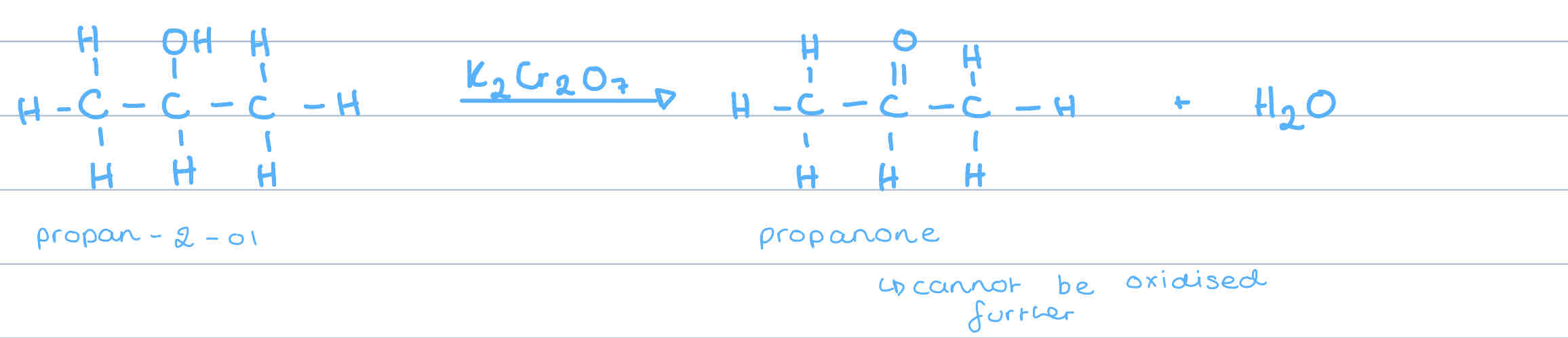
What do secondary alcohols oxidise to?
ketones
5
New cards
Why don’t ketones usually oxidise further?
due to the arrangement, no hydrogens are attached to the carbonyl group
6
New cards
Why is it hard to oxidise tertiary alcohols?
due to the arrangement, the carbon attached to the hydroxyl group isn’t attached to any other hydrogens