Orders, rate equations and rate constants
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
What does rate of reaction mean?
The change in concentration of a reactant or a product in a given time
What does order mean?
The power to which the concentration of a reactant is raised in a rate equation
What does overall order mean?
The sum of all the orders in a rate equation
What does rate constant mean?
The constant that links the rate of reaction with the concentrations of the reactants raised to the powers of their orders in the rate equation
What does half-life mean?
The time taken for the concentration of a reactant to decrease by half
What does rate-determining step mean?
The slowest step in the reaction mechanism of a multi-step reaction
When will the reaction be zero order with respect to the reactant?
When the concentration of a reactant has no effect on the rate
When is a reaction first order with respect to a reactant?
When the rate depends on its concentration raised to the power of 1
If the concentration is doubled, the rate doubles etc.
When is a reaction second order with respect to a reactant?
When the rate depends on its concentration raised to the power of two
If the concentration is doubled, the rate quadruples etc.
What does a rate equation look like?
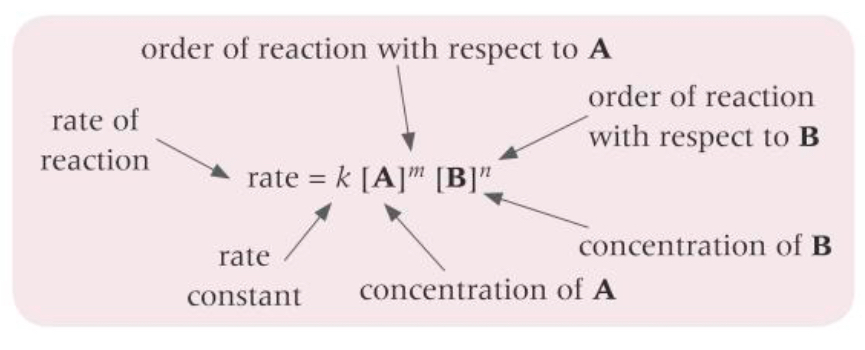
How do you work out the units of k?

The orders of reaction can only be determined by what?
Must be determined experimentally
When is half-life constant?
first-order reactions