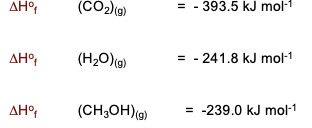FUNCHEM 25: Driving Forces Behind Chemical and Biological Reactions
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
What does thermodynamics and spontaneous reactions mean?
Thermodynamics- tool used to predict whether a reaction is spontaneous or not
Spontaneous reaction: occur of their own accord
What are the driving forces behind chemical and biological reactions?
Enthalpy and Entropy
What is the symbol and unit of Enthalpy
H and kJ mol-1
In energy profile diagram would the arrow go up or down for exothermic and endothermic reactions
Down for exothermic
Up for Endothermic
What is Standard Enthalpy Change
Enthalpy change art temperature of 298K and 1atm pressure
What are the different types of enthalpy change? and Explain
Standard Enthalpy Change of Formation- is the enthalpy change that occurs when 1 mole of a compound is formed from its uncombined elements at 298K and 1 atm pressure
Standard Enthalpy Change of Combustion- is the enthalpy change that occurs when 1 mole of an element or compound has been burned in excess oxygen at 298 K at 1 atm
Standard Enthalpy Change of Reaction
What is the first law of thermodynamics
Energy cannot be created or destroyed, it can only be converted from one form to another
What does Hess’s law state?
Hess’s Law states that the enthalpy change. of a reaction is the same regardless of the pathway by which a reaction occurs.
What are the rules for thermochemical reactions?
Must have balanced reactions
Must specify the states of the reactants and products
Reverse reaction= reverse sign
DH depends on the amount reacting
Enthalpy of formation of an element in its most stable form
Determine the molar heat of combustion of methanol