graphene and fullerenes
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
what are graphene and fullerene molecules all based on?
carbon atoms
what is graphene and what does this mean?
a single layer of graphite this means it’s only one atom thick.
why is graphene a good conductor of electricity?
it has delocalised electrons, these delocalised electrons can move through the graphene molecule carrying electrical charge.
graphene is extremely strong and has a high melting and boiling point why?
because graphene has a large number of strong covalent bonds and these covalent bonds require a great deal of energy to break.
what will these properties make graphene useful for?
producing new materials
what shape do fullerene molecules have?
hollow shapes, they usually have hexagonal rings of carbon atoms.
fullerenes can also have rings with how many carbon atoms?
five or seven carbon atoms
what is the first fullerene molecule to be discovered called?
buckminsterfullerene
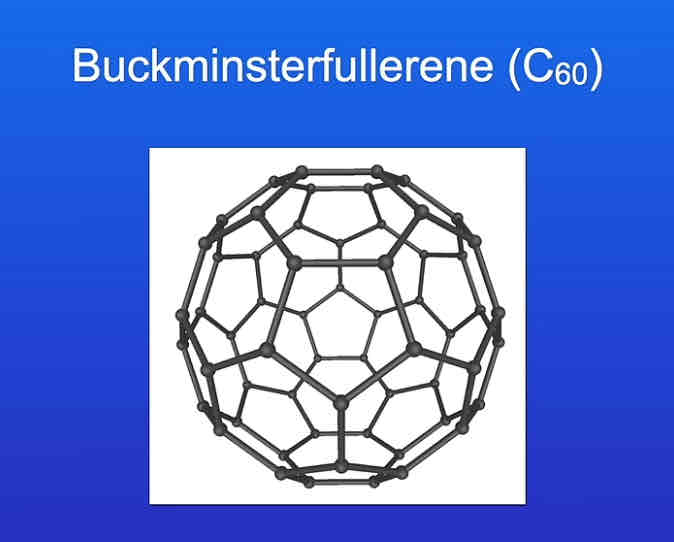
what does buckminsterfullerene contain?
sixty carbon atom arranged in a hollow sphere.
what do the carbon atoms form rings with?
either six carbon atoms of with five carbon atoms.
uses of fullerene
used to deliver drugs such as pharmaceuticals into the body.
they can be used as lubricants in machines where they reduce friction between moving parts.
used as catalysts to speed up chemical reactions.
what is one group of fullerenes called?
carbon nanotubes
what are carbon nanotubes?
these are fullerenes shaped into long cylinders with a relatively small diameter.
what do carbon nanotubes have a very high length to what ratio?
a very high length to diameter ratio.
carbon nanotubes have rings formed from?
six carbon atoms
useful properties of carbon nanotubes - high tensile strength
high tensile strength meaning we can apply a great deal of stretching force to a carbon nanotube before it breaks.
useful properties of carbon nanotubes - delocalised electrons
they have delocalised elections which makes them good conductors of electricity.
what are carbon nanotubes also good conductors of?
heat
what is one use of carbon nanotubes?
to reinforce materials.