CHEM 233 Stereochemistry
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
What are stereoisomers
same molecular formula, same connectivity, but different arrangements in space
What are constitutional isomers
same molecular formula, different connectivity
two types of stereoisomers,
configurational and conformational
two types of configurational isomers
diastereomers and enantiomers
Conformational isomer —> conformer, what is that?
an isomer of a molecule that differs from another isomer by the rotation of a single bond in the molecule
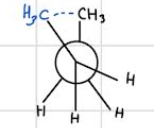
syn — eclipsed — highest in energy
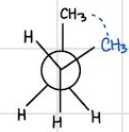
gauche — staggered (second lowest in energy)
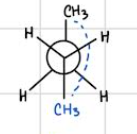
anti — staggered (lowest in energy)
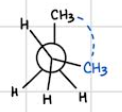
Eclipsed — second highest in energy
What are enantiomers
non-superimposable mirror images
identical physical and chemical properties
(except: interactions with plane polarized light)
What are diastereomers
not mirror images of each other
similar physical / chemical properties
E/Z stereoisomers
compounds with multiple assymetric centres
All assymetric centres are sterecentres, but…
not all stereocentres are assymetric centres
Meso Compound
more than 1 assymetric centre
internal plane of symmetry
What is a stereocentre
an atom (usually carbon) with at least three different groups attached, such that swapping any two of these groups creates a different stereoisomer