WBC - Granulocytes
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
60 Terms
What are the myeloid types of WBC
•Granulocytes
•Mononuclear phagocytes
List three granulocytes and two mononuclear WBCs
Granulocytes: Neutrophils, Eosinophils and Basophils
Mononuclear WBC - Lymphocytes and monocytes
What cytokines and what produces them stimulates production/maturation of neutrophils
IL-3 - T cells
GM-CSF - T cells, endothelial cells, monocytes and fibroblasts
G-CSG - Endothelial cells, placenta and monocytes
What cytokines and what produces them stimulates production/maturation of eosinophils
IL-3 - T cells
IL5 - T cells and basophils
GM-CSF - T cells, endothelial cells, monocytes and fibroblasts
What cytokines and what produces them stimulates production/maturation of basophils
IL3 - T cells
IL4 - B & T cells, eosinophils
GM- CSF T cells, endothelial cells, monocytes and fibroblasts
What are the normal ranges for Neutrophils, Lymphocytes, Monocytes, Eosinophils and Basophils
Normal [x 109/l] | |
Neutrophils | 1.8 – 7.5 |
Lymphocytes | 1.5 – 4.0 |
Monocytes | 0.2 – 0.8 |
Eosinophils | 0 – 0.4 |
Basophils | 0.01 – 0.1 |
Why would neutrophils be elevated
Bacterial infection, stress, exercise, myeloproliferative diseases e.g. leukaemia
Why would lymphocytes be raised
Viral infection, lymphoproliferative diseases (e.g. lymphocytic leukaemia)
Why would Monocytes be elevated
Infection, inflammation, tissue damage, monocytic leukaemia
Why would Eosinophils count be elevated
Allergy, intestinal parasites, hypereosinophilic syndrome, eosinophilic leukaemia
Why would Basophils be elevated
Some myeloproliferative diseases (esp. Chronic granulocytic leukaemia)
What are the main roles for neutrophils, eosinophils and basophils
Neutrophils – the elimination of invading bacteria and some fungi
Eosinophils – elimination of parasitic worms (=helminths), regulation of local immune and inflammatory responses
Basophils – Immune system regulation, secretion of heparin and histamine, allergy, inflammation, parasite defence, ? tumour surveillance
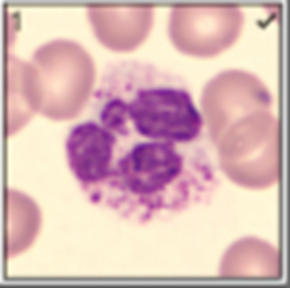
Describe the structure of a neutrophil
Neutrophils:
granules are
neutral-staining
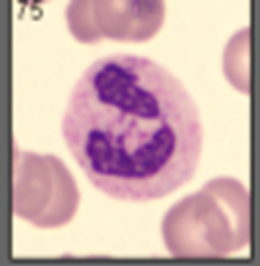
Describe the eosinophil structure
granules stain with eosin (orange)
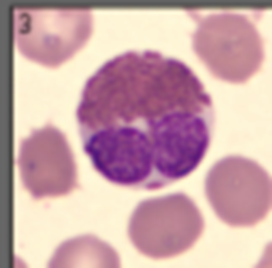
Describe a Basophils structure
granules stain intensely with methylene blue
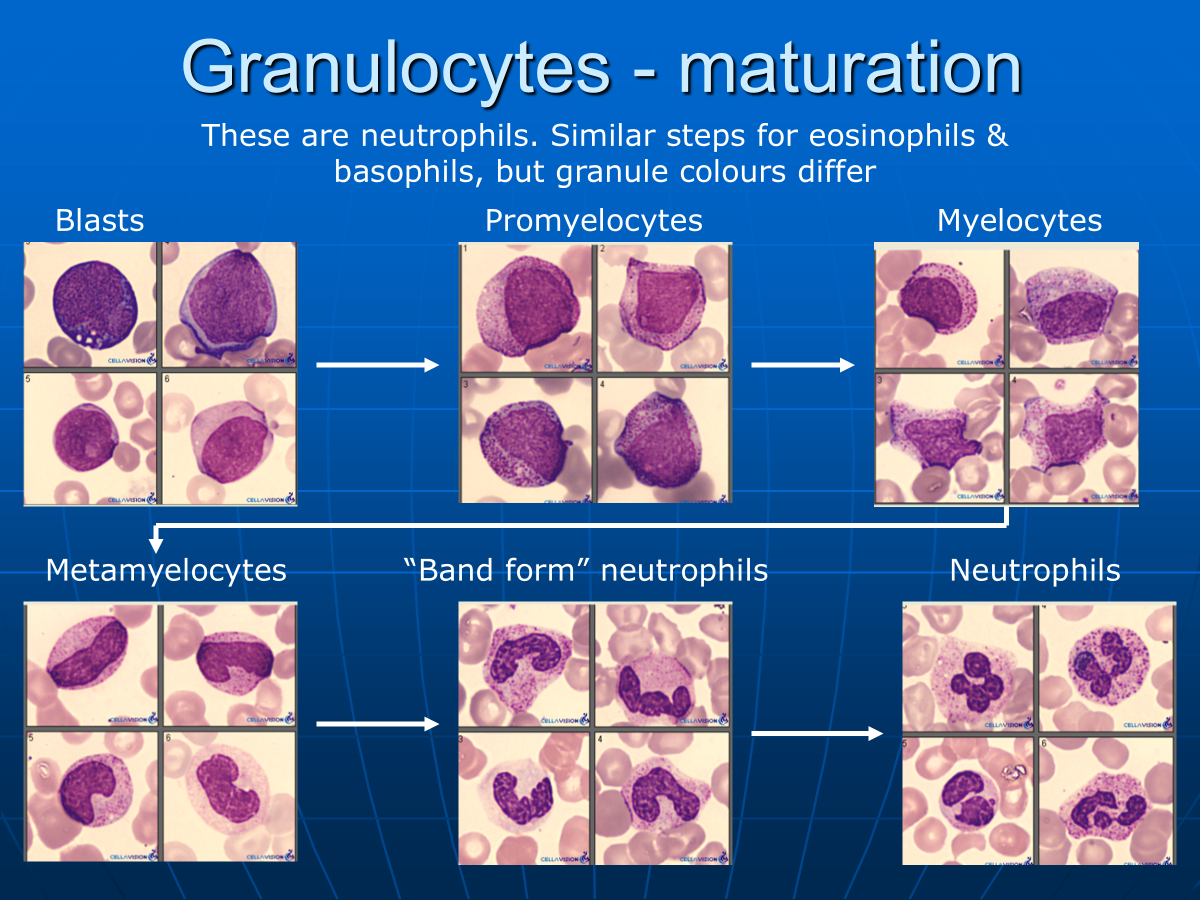
What are the stages of neutrophil maturation
What is the granulocyte turnover
50 – 320 x 109/day
(= approx 370,000 / sec)
How long does neutrophils spend in the peripheral blood and then tissues
Peripheral blood ~ 7 hours
Tissues ~ 20 hours
What is the lifespan of an eosinophil
8-12 hours in circulation then 8 – 12 days in tissues (thymus, lower GI tract, ovary, uterus, spleen and lymph nodes)
What is the lifespan of basophils
2-3 days in circulation then die
Why is a neutrophils nucleus lobulated
To aid deformability and motility
What are the granules present in neutrophils
•Primary – discharge into phagosomes. Contain microbicidal proteins (eg Myeloperoxidase, hydrolases & lysozyme) for oxidative and non-oxidative killing. Main function is killing and digesting micro-organisms.
•Secondary – discharge into phagosomes and extracellularly. Contain e.g. hydrolases, alkaline phosphatase, lysozyme and collagenase.
•Tertiary – Contain e.g. alkaline phosphatase, gelatinase (involved in the destruction of collagen), cathepsin (a protease)
What does the neutrophils function involve
Location by chemotaxis and then phagocytosis
How does chemotaxis work
Vessel wall adhesion. Tissue damage > neutrophil adhesion to endothelial cells of vessel wall > migration into tissues. Adhesion using adhesive membrane receptors.
Movement up a concentration gradient. Chemo-attractants bind to specific neutrophil surface receptors, e.g. complement components, esp C5a, bacterial metabolic by-products, leukotriene B4 (an inflammatory mediator, made by monocytes/macrophages). Neutrophil receptors can detect concentration changes as small as 2%.
What are the 4 steps of phagocytosis
1) Requires opsonisation (i.e. coating) of particle with e.g. IgG or IgM antibodies or complement.
Neutrophils require particle to be completely coated with opsinin for successful phagocytosis
2) Particle attaches to neutrophil via a receptor for the opsonin
3) Pseudopodia enclose particle, which is ingested > phagosome
4) Neutrophil granules fuse into a phagosome and release contents, killing invading organism.
What is a respiratory burst
Increase in O2 consumption
Increase in glycolysis
Uprating of bactericidal processes e.g. Myeloperoxidase (MPO) activity
Increased expression of some constituents, e.g. Alkaline phosphatase
How does microbial killing usually work

What are some disorders of phagocytosis and how do they work
1) Myeloperoxidase deficiency.
Fairly common. Partial or total. Only 20% of patients are
immunocompromised. Oxygen free radicals (O-) & lysozyme
compensate. Fungal infections are the biggest problem.
2) Respiratory burst failure – esp NADPH oxidase
Inherited, metabolic failure of microbial killing, affects 1 in 200,000. (= Chronic Granulomatous Disease (CGD).
Some organisms live in the phagosome > persistent infections.
Multiple granulomas (accumulations of immune system cells).
Non-oxidative killing partially compensates
3) Inherited defects in neutrophil adhesion / migration are very rare.
Acquired defects, e.g. leukaemia, diabetes, renal failure, > varying degrees of susceptibility to sepsis.
Other neutrophil disorders
Neonate neutrophils have only 20 – 27% chemotactic activity of adults , less in premature neonates.
Neutrophil function declines with age – chemotaxis and phagocytosis significantly impaired in the elderly.
Abnormal neutrophils (e.g.hypogranular or agranular) are found in myelodysplasia (“pre-leukaemia”), common in the elderly and in leukaemia
What are some features of chronic granulocytic leukaemia
Increased and unregulated
growth of myeloid cells in
bone marrow.
Increased myeloid cell
numbers in blood, many
early (but mainly
differentiated) forms evident.
Many cases now “cured” by
tyrosine kinase inhibitor
therapy eg Imatinib
(in cases of 9:22 translocation which deregulates tyrosine kinase activity)
What indicates incorrect function of neutrophils
Chronic bacterial infection
Increased susceptibility to bacterial infections
Therapy-resistant infections
Recurrent infections with nonpathogenic microorganisms
Abscesses of liver or lung
What is NBT dye reduction and whats it for
Nitroblue tetrazolium for chronic granulomatous disease
What does NBT test for
Functional test of neutrophil respiratory burst (production of active oxygen species e.g. O-). Reduction of NBT to an insoluble blue compound by active neutrophils. Visual assessment (microscopy) of results.
Largely superceded by direct measurements of respiratory burst products using flow cytometry
How is neutrophil motility tested
Motility by assessing ability to penetrate a filter membrane or observed movement across a glass slide
How is neutrophil phagocytosis tested
•Ingestion, e.g. by observing reduction in the number of free bacteria in a bacteria + neutrophil suspension
•Killing, e.g. by observing the intra-cytoplasmic fall in numbers of phagocytosed bacteria.
Where are Eosinophils usually found
Thymus, lower GI tract, ovary, uterus, spleen and lymph nodes only in lungs in airborne allergy
What is more numerous tissue or blood eosinophils
Tissue eosinophils are several 100 x more numerous than blood eosinophils
What are eosinophils function
Functions – key mediators of allergic inflammation and elimination of parasitic worm infections by antibody-dependant cell-mediated toxicity (IgE)
What does Eosinophils cationic protein do
creates pores in the membranes of target cells allowing potential entry of other cytotoxic molecules to the cell & has anti-viral activity
What is Major Basic Protein
(toxic to parasites & epithelial cells, causes release of histamine & heparin from basophils & mast cells)
What causes Eosinophils significant local tissue damage
Eosinophils degranulation
What Interleukin does Eosinophils produce
IL-4
•Important immunoregulatory cytokine
•Affects inflammation, B-cell activation, antibody production
What is hypereosinophilic syndrome
Defined as sustained unexplained eosinophilia > 1.5 x 109/l > 6 months (normal range 0-0.4)
A monoclonal population of activated T lymphocytes may be found, producing excess IL5 (so the primary problem is lymphoid in origin)
What is hypereosinophilic syndromes affect on organs
Causes organ dysfunction due to eosinophilic infiltration (heart, lungs, GI tract, spleen, skin, CNS, etc)
What does treatment of hypereosinophilic syndrome aim to do and what is the treatment
Treatment attempts to limit organ damage by control of eosinophils using steroids, hydroxyurea, chemotherapy (Imatinib) therapy.
Is hypereosinophilic syndrome a fatal disorder
Most cases are eventually fatal but 80% survival to 5 years
What are the differences between basophils and mast cells
Treatment attempts to limit organ damage by control of eosinophils using steroids, hydroxyurea, chemotherapy (Imatinib) therapy.
What activates basophils
Basophils are activated by e.g. IgE, IL-3, C5a, GM-CSF & insect venoms to release granule contents, especially histamine.
What do basophils mediate
Basophils are key mediators of immediate hypersensitivity reactions e.g. asthma, urticaria & anaphylaxis
There is also possible evidence they supress tumour growth
List what basophils secrete and the function of each secretion
•Histamine - chemotactic agent for eosinophils & is a vasodilator
•Heparin – anticoagulant
•IL-3, a basophil growth factor, regulates macrophage and granulocyte populations in inflammation.
•IL-4, attracts eosinophils and supports B-cell activation
•IL-5, basophil and eosinophil production and activation, B-cell growth and activation
•IL-13, promotes B-cell proliferation and differentiation
•TNF-α –pro-inflammatory cytokine
•GM-CSF –inflammation-associated cytokine, activates neutrophils, moderates monocyte activation and is a growth and differentiation factor for granulocytes and macrophages
What causes significant basophilia
Significant basophilia is common in Chronic Myeloid Leukaemia but otherwise rare. (Suggestions that basophil numbers correlate with plasma triglyceride levels are probably counting artefacts giving a falsely elevated count).
Basophilia to a lesser extent is found in other myeloproliferative disorders, e.g. myelofibrosis & Primary Proliferative Polycythaemia.
Why is treatment for basophil leukaemia difficult
It is a rare disorder and it’s difficult due to release of histamine and other granule contents
How are basophils tested
basophil Activation Test (BAT).
Assesses the expression of activation markers (CD63 or CD 203c) on the surface of live basophils or measures the degree of basophil degranulation.
In whole fresh blood by flow cytometry following stimulation with allergen
Usually in investigation of peanut allergy, using peanut allergen
Distinguishes between allergic and tolerant individuals
NB Performed exceptionally rarely (antigen challenge instead)
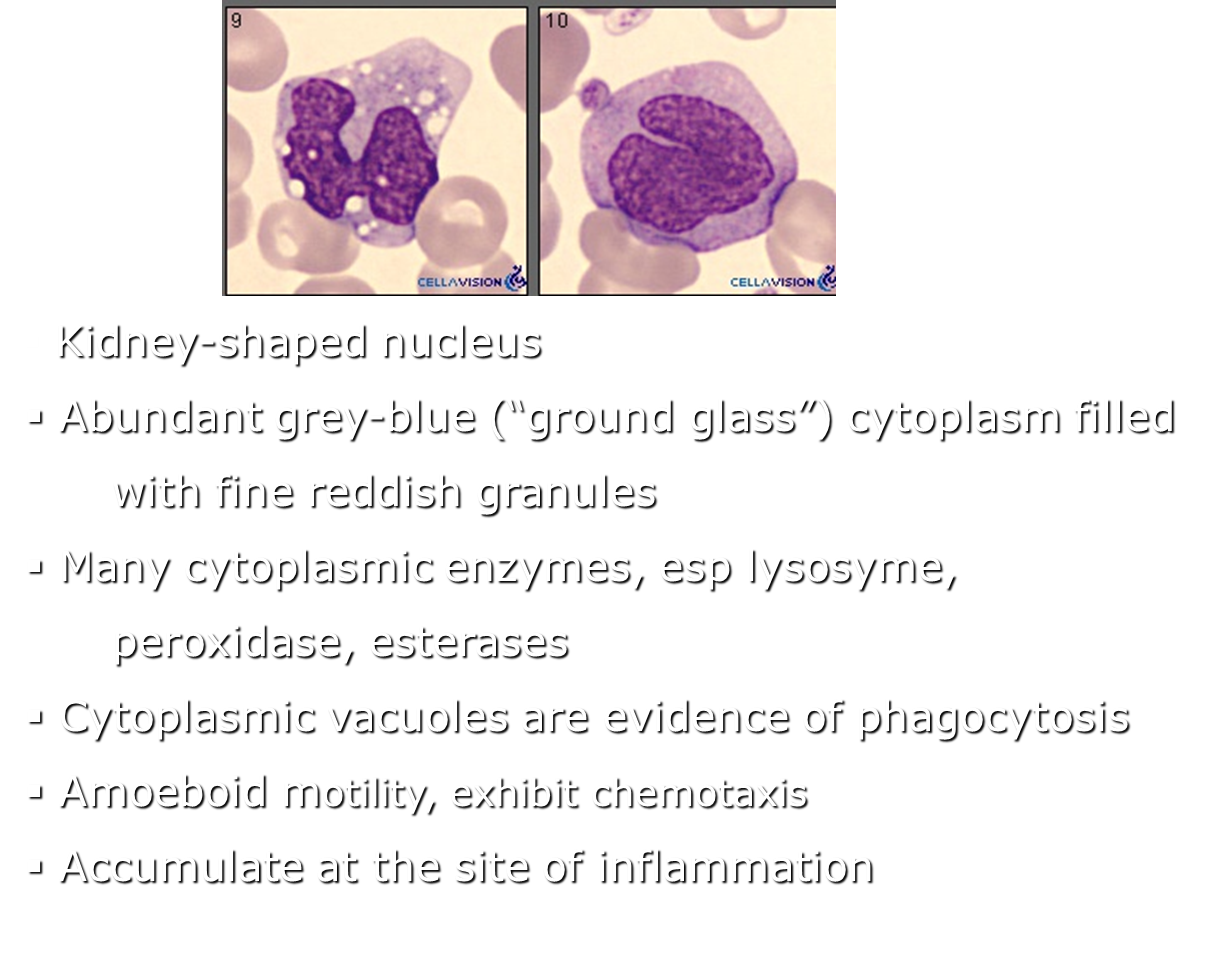
Describe a monocytes structure and contents
What are the function of monocytes
Phagocytosis
Antigen presentation (to T cells)
Cytokine production
Differentiation into macrophages and monocyte-derived dendritic cells
Critical role in both innate and adaptive immunity (phagocytic WBCs are innate only)
How can monocytes bind to endothelial cells
With different adhesive glycoproteins which facilitate adhesion
What cytokines do monocytes release
Release cytokines e.g. Pro-inflammatory IL1, IL6, IL8, G-CSF, Tumour Necrosis Factor-α (TNF-α, involved in cell signalling, apoptosis, tumour cell suppression )
What is the lifespan of monocytes
Most in circulation are short-lived (24h)
As macrophages and dendritic cells live for months or years
What causes an increased number of monocytes
in chronic infections and inflammatory conditions, e.g. tuberculosis & Crohn’s disease
What are some examples of inherited impairment of degradation of phagocytosed material
“Lipid storage diseases”, e.g. Gaucher’s disease, Niemann-Pick disease result from an accumulation of debris within macrophages
Cause permanent cellular and tissue damage, particularly in the brain, peripheral nervous system, liver, spleen, and bone marrow.