Introduction to Thermodynamics Physical Chemistry
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
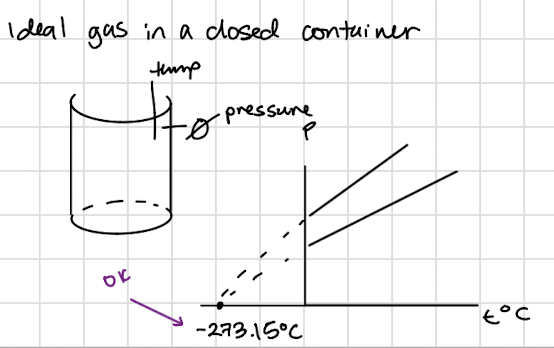
Temperature
when a hotter and a colder bodies are brought in contact, heat flows from the hotter body to the colder body.
at thermal equilibrium, two bodies in contact have equal temperatures and there is not net heat flow from one body to the other.
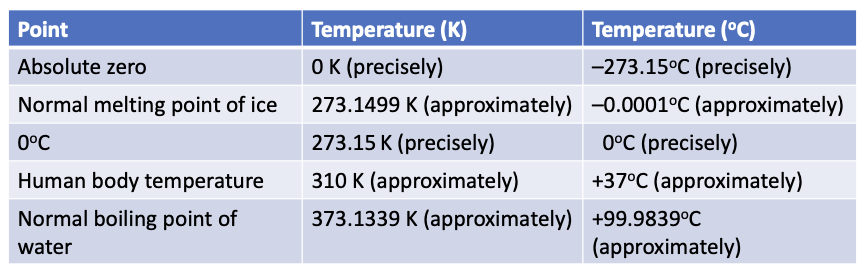
in thermodynamics, there is the lowest temperature (the absolute zero, T=0-the absolute scale of temperature)
SI unit: 1 K
kB=1.38064852×10-23 J/K
∆1˚C=∆1K
Pressure
P=F/A
SI unit: 1Pa = 1N/m2
1 bar = 105 Pa
1 atm = 101325 Pa = 1.01325 bar
1 mmHg = 1 torr
1 atm = 760 torr
mechanical equilibrium p1=p2
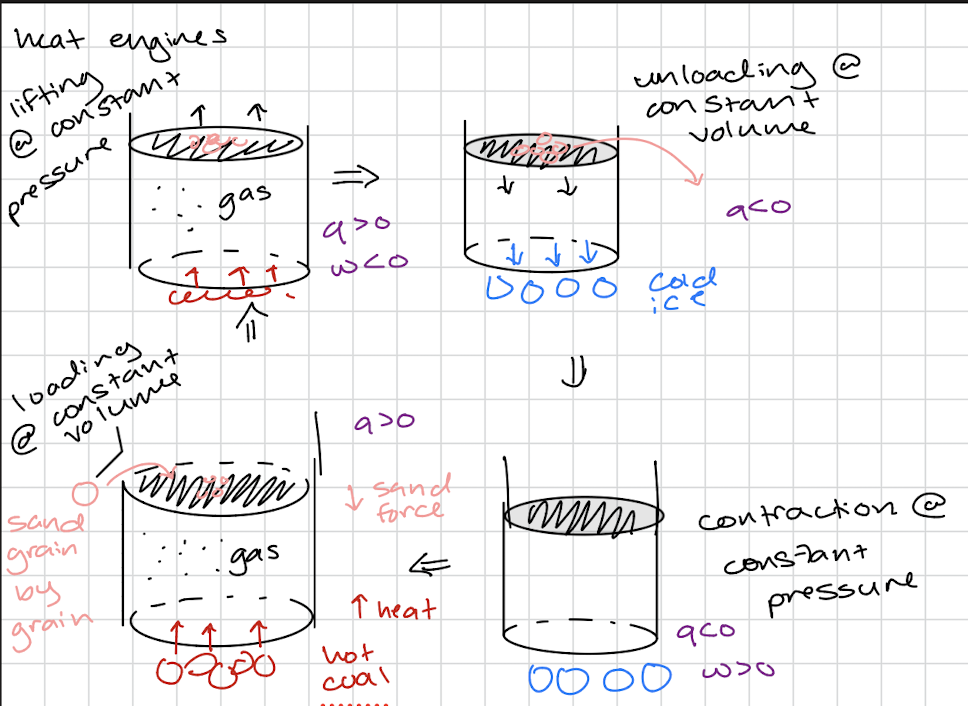
Work
transfer of energy that causes uniform motion of atoms in the surroundings
always specify the conditions (const T, p, or const T, V)
Heat
transfer of energy that causes random motion of atoms in the surroundings
always specify the conditions (const T, p, or const T, V)
Open System
Allow exchange of energy and matter
Closed System
Allow exchange of energy but NOT matter
Isolated System
No exchange of energy or matter
The Universe
= the system+ the surroundings
What kind of system is a biological cell?
Open System
Sign Convention
(Point of view of the system)
Heat absorbed by the system q>0 endothermic
Heat released from the system q<0 exothermic
Work done on the system w>0
Work done by the system w<0
Diathermal Wall
Allows for heat transfer
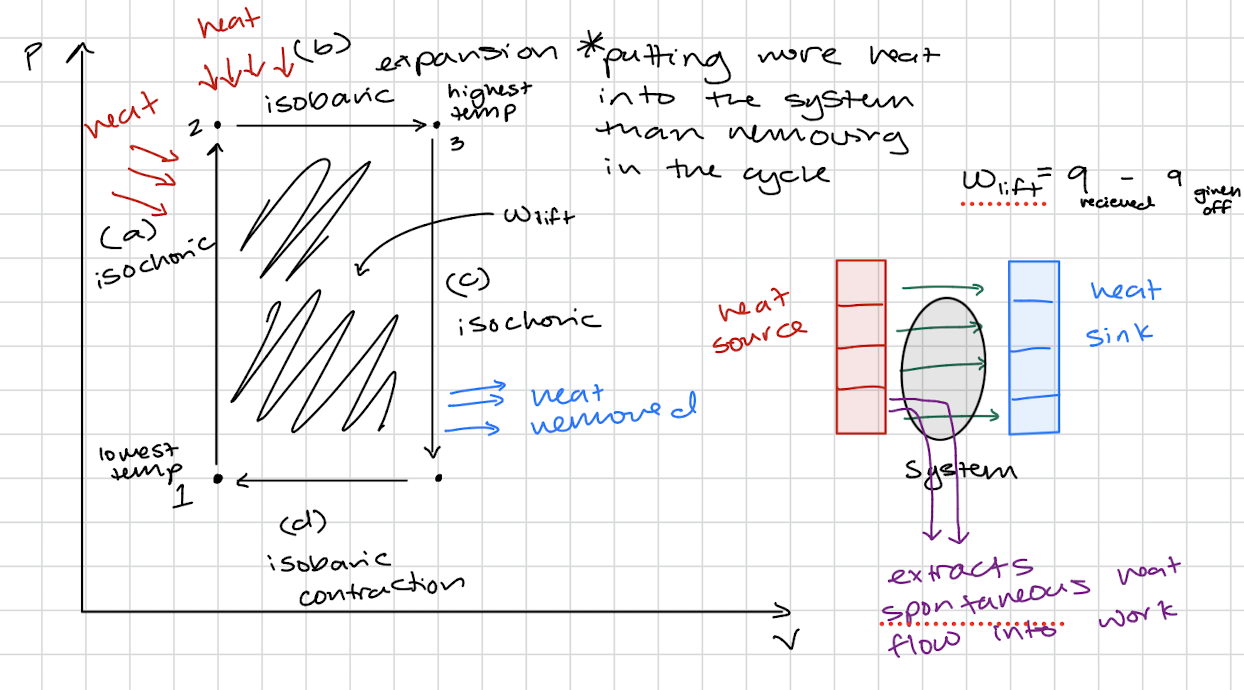
Heat Engines
Heat Engine P vs V graph
Isothermal Process
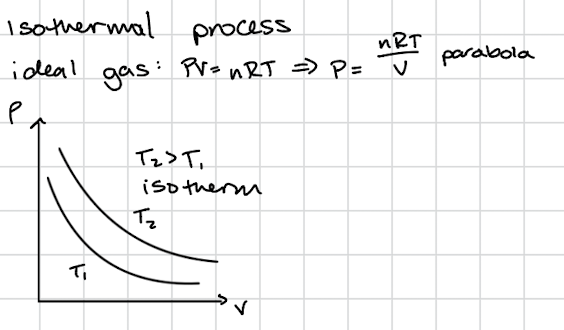
Absolute Zero