7.3, 7.4 Reversible Reactions, Equilibrium
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
Forward will form
products
Backward will form
reactants
Define water of crystallisation
water incl in structure of salts during crystallisation
Consider copper(II) sulfate pentahydrate:
CuSO4.5H2O(s) + heat ⇌ CuSO4(s) + 5H2O(l)
CuSO4.5H2O is heated & loses water of crystallisation, forming the anhydrous salt (CuSO4)
This can be reversed by adding __
Forward is e__ as e__ is needed to remove water
water, endo, energy
Define dynamic equilibrium
rate forward = rate backward in closed system
Define closed system
nothing can be added/removed to system except energy
Le Chatelier’s Principle
> if c__ is made to s__ in e__, the eq s__ in the d__ that will __ the change
change, system, equilibrium, shifts, direction, minimise
Factors Affecting Equilibrium Position
C__
> ↑C of reactants = eq shifts __ = __(more/less) yield
conc, right, more
Factors Affecting Equilibrium Position
P__
> ↑P = e__ pos moves to __(least/most) amt of moles
pressure, eq, least
Factors Affecting Equilibrium Position
T__
> ↑T = eq pos moves to e__
> system counteracts change (too high temp) by a__ heat
temp, endo, absorbing
Factors Affecting Equilibrium Position
C__
> only (increases/decreases) time for reaction to reach eq
catalyst, decreases
Do catalysts shift equil pos?
no
What is ammonia used for?
fertiliser
Conditions for Haber Process
__C, __atm, w/ __ catalyst = ~__% ammonia yield
450, 350, iron, 15
Why These Conditions for Haber Process?
> are a c__ for yield, rate, safety, cost
> lower temp = too s__
> pressure is s__ enough & equipment is e__
> catalyst s__ up reaction = yield achieved at lower temp
compromise, slow, safe, expensive, speeds
Haber process sources
H2 from n__ gas; N2 from the __
natural, air
Contact process makes what?
sulfuric acid
Sulfuric acid is used in: c__ batteries, fertilisers, s__ & detergents
car, soaps
Contact Process
1. Make s__ d__ via burning s__ in a__
S(s) + O2(g) → SO2(g) + heat
sulfur dioxide, sulfur, air
Contact Process
2. O__ sulfur d__ → sulfur t__ w/ v__ o__ (__2O5) catalyst
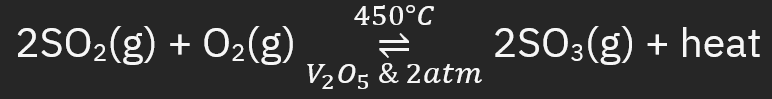
oxidate, dioxide, trioxide, vanadium oxide, V
Contact Process
3. Absorb sulfur t__ into sulfuric a__, making o__ (disulfuric acid)
> SO3(g) + H2SO4(l) H2S7O7(l) + heat
trioxide, acid, oleum
Contact Process
4. Add o__ to w__, making sulfuric acid
> H2S7O7(l) + H2O(l) 2H2SO4(l)
oleum, water
Contact process conditions
__C, __atm
450, 2
Why these contact process conditions?
> temp: too low = too s__
> pressure: too h__ = l__ SO2 & e__ equipment & dangerous
slow, high, liquefies, expensive
Sulfuric acid also acts as a d__ agent (removes water)
dehydrating
Conc. sulfuric acid contains molecules, not ions. thus is a (poor/good) conductor of electricity
poor
Dilute sulfuric acid contains ions & is aq. thus is a (poor/good) conductor of electricity
good