Chem Chapter 6
1/110
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
111 Terms
chemical bond
mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together
Nature aims to minimize potential energy
Atoms bond to achieve a more stable state by lowering their potential energy
Ionic Bonding occurs between what types of elements?
Metal and Nonmetal
In ionic bonding what happens to the electron?
It is transferred from the metal to the nonmetal
What happens to the charge of the metal in ionic bonding?
It loses an electron and becomes positively charged
What happens to the charge of the nonmetal in ionic bonding?
It gains an electron and becomes negatively charged
Covalent bonding
occurs between non-metals, sharing of electrons
Nonpolar Covalent definition
equal sharing of electrons
Polar covalent definition
unequal sharing of electrons
Nonpolar covalent E.N difference
0-0.3
Polar covalent E.N difference
0.3-1.7
Nonpolar covalent percentage ionic charecter
0%-5%
Polar covalent percentage ionic charecter
5%-50%
Polar
uneven distribution of charges
Octet rule
States that atoms lose, gain or share electrons in order to acquire a full set of eight valence electrons
Incomplete octet
stable with fewer than eight electrons
Expanded octet
when a molecule has more than 8 electrons around the central atom
Empirical formula
chemical formula that gives the simplest whole number ratio of atoms in a compound (lowest terms) Ex. C₆H₁₂O₆ → CH₂O
Hydrogen (H) octet exception
only needs 2 electrons to be full
Helium (He) octet exception
only needs 2 electrons to be full
Beryllium (Be) octet exception
only needs 4 electrons to be full
Boron (B) octet exception
Only needs 6 electrons to be full
Lithium (Li) octet exception
Forms Li⁺ ion with 2 electrons
Aluminimum (Al) octet exception
Sometimes stable with 6 electrons
Phosphorus (P) octet exception
Can have 10 electrons
Sulfur (S) octet exception
Can have 12 electrons
ionic compound properties
high MP (solid at room temp); poor conductors in solid state; good conductors when melted or dissolved; hard and brittle
Ionic compound
composed of positive and negative ions that are combined so that the numbers of positive and negative charges are equal
Formula unit
the simplest collection of atoms from which an ionic compound's formula can be established (Ex. NaCl)
Crystal lattice
A 3-dimensional geometric arrangement of the atoms or molecules or ions composing a crystal to minimize potential energy
Lattice energy
the energy released when one mole of an ionic crystalline compound is formed from gaseous ions (negative values indicate release)
polyatomic ion
A charged group of covalently bonded atoms
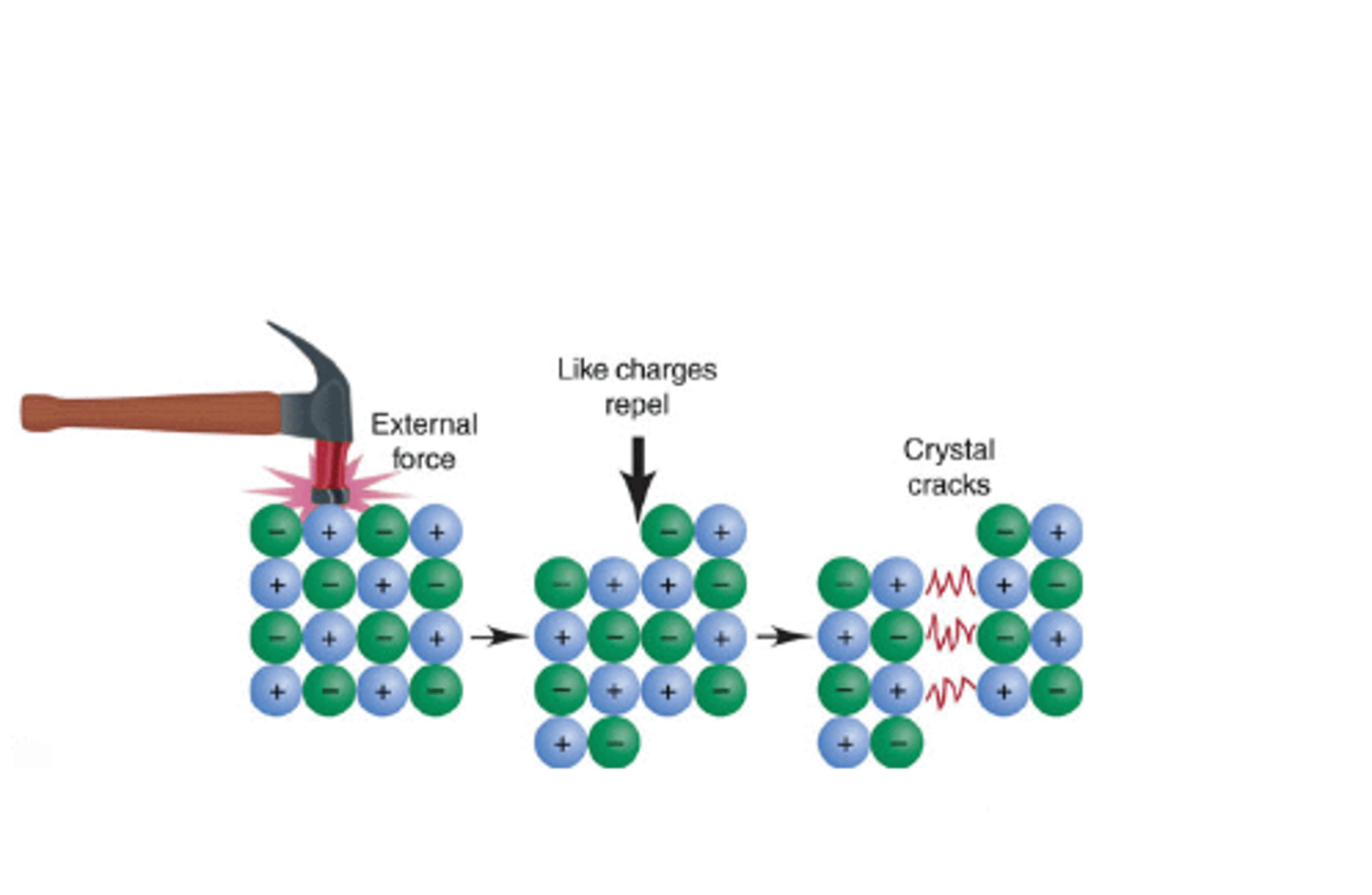
Why are ionic compounds hard but brittle?
The strong attraction between positive and negative ions holds them in a rigid, tightly packed arrangement. A large amount of energy is required to break these forces, making ionic compounds hard. If the layers move however, repulsion forces compete leading to a shattering of the layers
Delocalized electrons
electrons that are free to move through vacant orbitals
molecule
A group of atoms bonded together covalentently
molecular compound
a chemical compound whose simplest units are molecules
chemical formula
shows the elements in the compound and the ratio of atoms. Used for both ionic and molecular compounds.
molecular formula
a chemical formula of a molecular compound that shows the kinds and numbers of atoms present in a molecule of a compound
bond length
the average distance between the nuclei of two bonded atoms
bond energy
the energy required to break a chemical bond and form neutral isolated atoms
resonance
the bonding in molecules or ions that cannot be correctly represented by a single Lewis structure represneted with <--->
sea of electrons model
Simplified description of metallic bonding in which the valence electrons of metal atoms are delocalized and move freely throughout the solid rather than being tied to any specific atom.
enthlapy of vaporization
the amount of heat energy required to vaporize a metal
Why are metals good reflectors of light?
their mobile "sea of electrons", which can absorb and re-emit a wide range of light frequencies. When light strikes a metal surface, the free electrons oscillate and immediately release the absorbed energy as reflected light.
Why are metals good conductors of heat and electricity?
Metals conduct electricity because their free electrons move easily when a voltage is applied. They conduct heat as these electrons transfer energy quickly across the structure.
Strengh of metallic bonds depend on
number of valence electrons that can be delocalized, the charge of a metal ion(higher charge = higher strength), and the ionic radius of a metallic cation.
Why are metals malleable and ductile?
The layers of atoms in a metal can slide over each other
VSEPR theory
Valence-shell electron-pair repulsion theory; because electron pairs repel, molecules adjust their shapes so that valence electron pairs are as far apart as possible
Linear - Atoms bonded to central atom
2
Linear - Lone pairs of electrons
0
Linear - Type of molecule
AB₂
Trigonal-planar - Atoms bonded to central atom
3
Trigonal-planar - Lone pairs of electrons
0
Trigonal-planar - Type of molecule
AB₃
Bent or Angular (1 lone pair) - Atoms bonded to central atom
2
Bent or Angular (1 lone pair) - Lone pairs of electrons
1
Bent or Angular (1 lone pair) - Type of molecule
AB₂E
Tetrahedral - Atoms bonded to central atom
4
Tetrahedral - Lone pairs of electrons
0
Tetrahedral - Type of molecule
AB₄
Trigonal-pyramidal - Atoms bonded to central atom
3
Trigonal-pyramidal - Lone pairs of electrons
1
Trigonal-pyramidal - Type of molecule
AB₃E
Bent or Angular (2 lone pairs) - Atoms bonded to central atom
2
Bent or Angular (2 lone pairs) - Lone pairs of electrons
2
Bent or Angular (2 lone pairs) - Type of molecule
AB₂E₂
Trigonal-bipyramidal - Atoms bonded to central atom
5
Trigonal-bipyramidal - Lone pairs of electrons
0
Trigonal-bipyramidal - Type of molecule
AB₅
Octahedral - Atoms bonded to central atom
6
Octahedral - Lone pairs of electrons
0
Octahedral - Type of molecule
AB₆.
intermolecular forces
electrostatic interactions between molecules
dipole
a molecule that has two poles, or regions, with opposite charges
ion-ion
ionic molecule + ionic molecule, strongest intermolecular force
ion-dipole
the charge of an ion is attracted to the partial charge on a polar molecule (strongest dipole intermolecular force)
dipole-dipole
attractions between oppositely charged regions of polar molecules
hydrogen bonding
strongest type of intermolecular dipole-dipole attraction. Occurs between hydrogen and F, O or N
London dispersion forces
interaction between a momentary dipole and an induced dipole, caused by the random movement of electrons.
momentary dipole
a momentary lopsided electron cloud
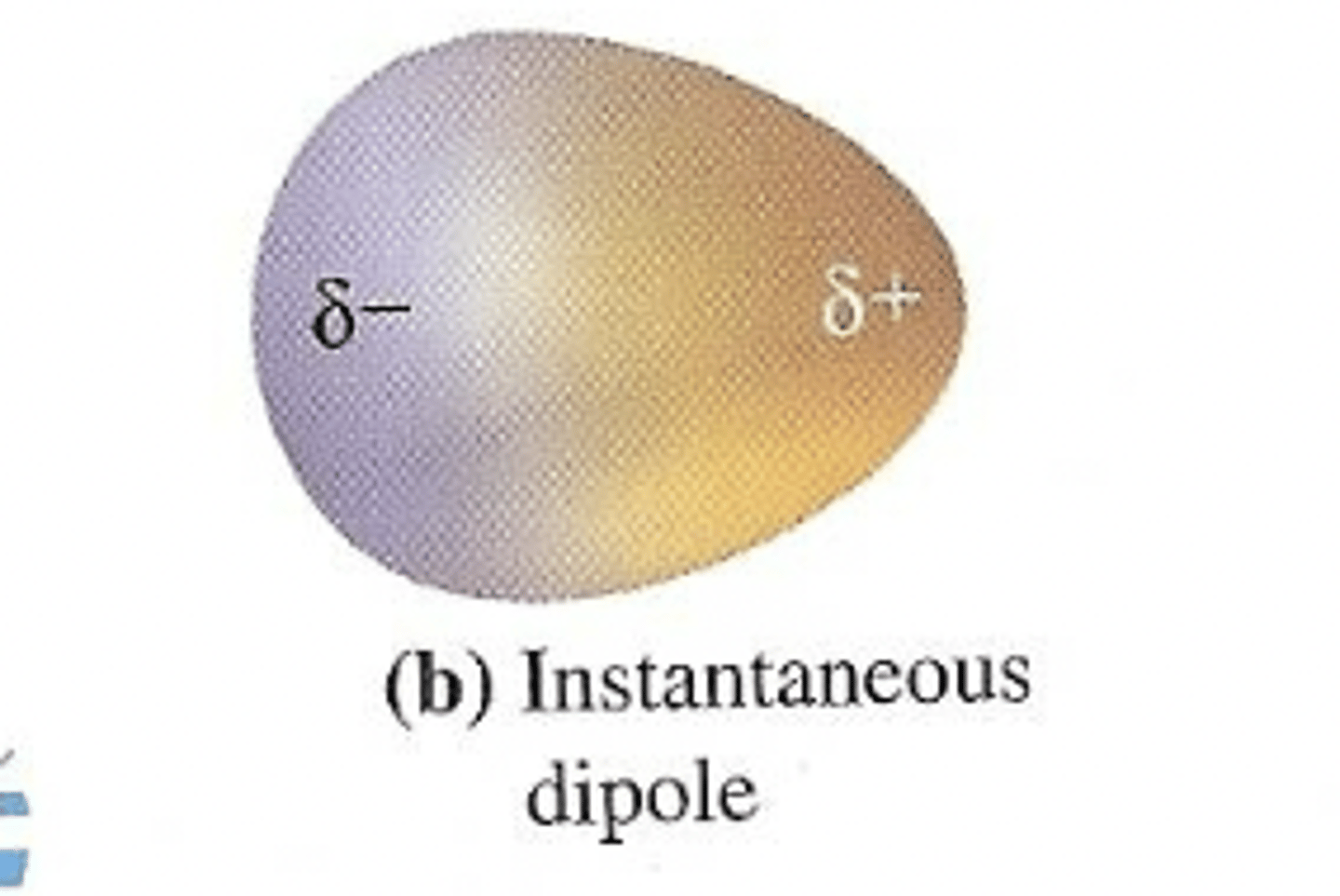
induced dipole
caused when a momentary dipole comes near another electron cloud
nonpolar covalent intermolecular force
London Dispersion forces
ionic bond intermolecular force
ion-ion
Linear VSEPR Diagram
180 degrees
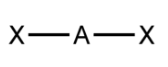
Trigonal-planar VSEPR Diagram
120 degrees
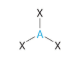
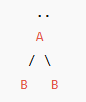
Bent or angular (1 lone pair) VSEPR Diagram
120 degrees
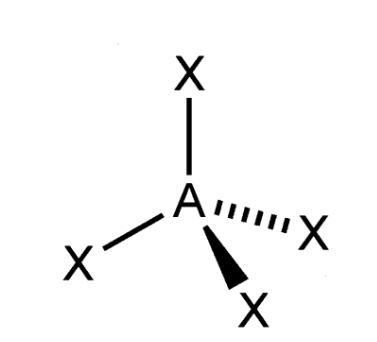
Tetrahedral VSEPR Diagram
109.5 degrees
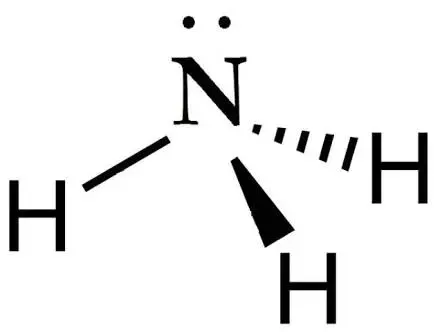
trigonal-pyramidal VSEPR Diagram
107 degrees
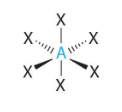
Octahedral VSEPR Diagram
90 degrees
Bent or angular (2 lone paris) VSEPR Diagram
104.5 degrees
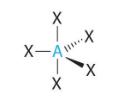
Triognal Bipyramidal VSEPR Diagram
90 and 120 degrees
hybridization
mixing of atomic orbitals of similar energies on the same atom to produce new hybrid atomic orbitals of equal eneriges.
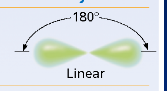
How many hybrid orbitals are formed in sp hybridization?
2 hybrid orbitals.
What is the molecular geometry of sp hybridization?
Linear, with a bond angle of 180 degrees.
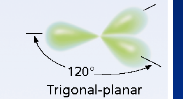
How many hybrid orbitals are formed in sp² hybridization?
3 hybrid orbitals.
What is the molecular geometry of sp² hybridization?
Trigonal planar with a bond angle of 120 degrees.
How many hybrid orbitals are formed in sp³ hybridization?
4 hybrid orbitals.
What is the molecular geometry of sp³ hybridization?
Tetrahedral with a bond angle of 109.5 degrees.
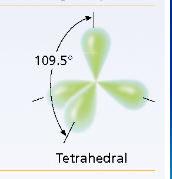
hybrid orbital
orbitals of equal energy produced by the combination of two or more orbitals on the same atom.
Ionic E.N Difference
1.7-3.3