chemistry - esters
1/13
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
what are esters formed from?
an alcohol and a carboxylic acid with the presence of an acid catalyst
what is the by product formed when an ester is formed?
water
what is the functional group of an ester?
-COO-
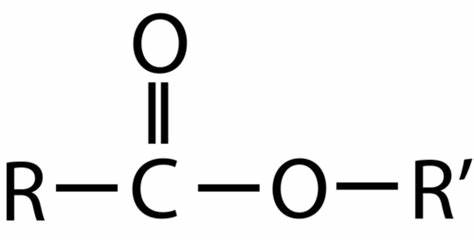
what do the names of esters end in?
-oate
how are the names of esters formed?
alcohol prefix + yl carboxylic acid prefix+ anoate
what ester would the reaction of ethanol and ethanoic acid form?
ethyl ethanoate
how do you draw the displayed formula of an ester?
cut off the -O-H group from the acid
cut off the final -H from the alcohol (have OH facing left)
squish together:
acid on left, alcohol on right
how do you write the structural formula of an ester?
acid first, then alcohol
CH3CH2…COOCH2…CH3
(H for methanoic acid)
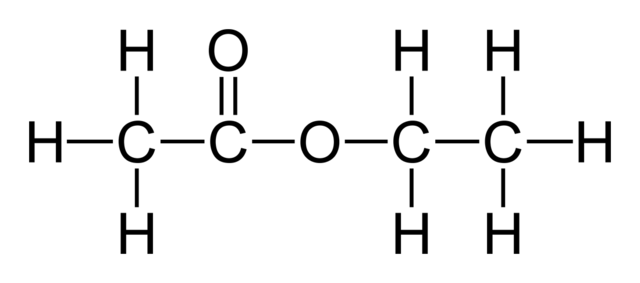
what is the structural formula of ethyl ethanoate?
CH3COOCH2CH3
what is the displayed formula of ethyl ethanoate?
how would you prepare an ester in a lab?
add a few drops of concentrated sulfuric acid to a boiling tube using a pipette
add 1cm3 ethanoic acid and the same volume of ethanol
place the tube in a beaker of 80C water for 5 minutes
pour the mixture into a test tube of sodium carbonate solution and mix
a layer of ester should form on top
what are the properties of esters?
fruity smells
sweet smells
volatile - evaporate easily
what are the uses of esters?
perfumes
flavourings