ACS Exam - OChem Final
1/167
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
168 Terms
Chapter 1: Structure: Shape and Stability
Covalent
Covalent bonds form between two nonmetals, usually from the right side of the periodic table (Groups 14–17),
Ionic Bond
Ionic bonds typically form between elements that are very far apart on the periodic table — usually between:
A metal from the left side (Groups 1–2, like sodium or calcium)
And a nonmetal from the right side (Groups 16–17, like oxygen or chlorine)
Molecular vs Empirical Formula
A molecular formula shows the actual number of atoms of each element in a molecule, whereas an empirical formula shows the simplest whole-number ratio of the atoms in the compound.
Filling Orbital Diagrams
All up arrows then fill down arrows. Also all lower levels should be filled before filling upper levels.
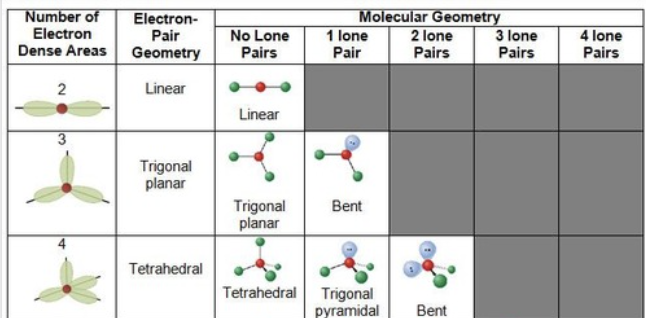
VESPR
Linear: 180 degrees; Trigonal planar: 120 degrees; Tetrahedral: 109.5 degrees.
Trigonal Planar if 3 atoms with no lone pairs surround the central atom (BH3)
Tetrahedral surrounded by 4 atoms and no lone pairs
Trigonal Pyramidal: 3 attached to the central atom with one lone pair
Linear consists of two atoms with no lone pairs around 107 degrees
Bent shape consists of 2 atoms and one or more lone pairs around the central atom, resulting in bond angles less than 120 degrees.
If asks for hybridization…. (BEFORE VS AFTER)
AFTER means 1 row
BEFORE means 2 rows
Intensity of Dipole Moment
Less symmetry = stronger dipole
Chapter 2 - Structure: Nomenclature and Functional Groups
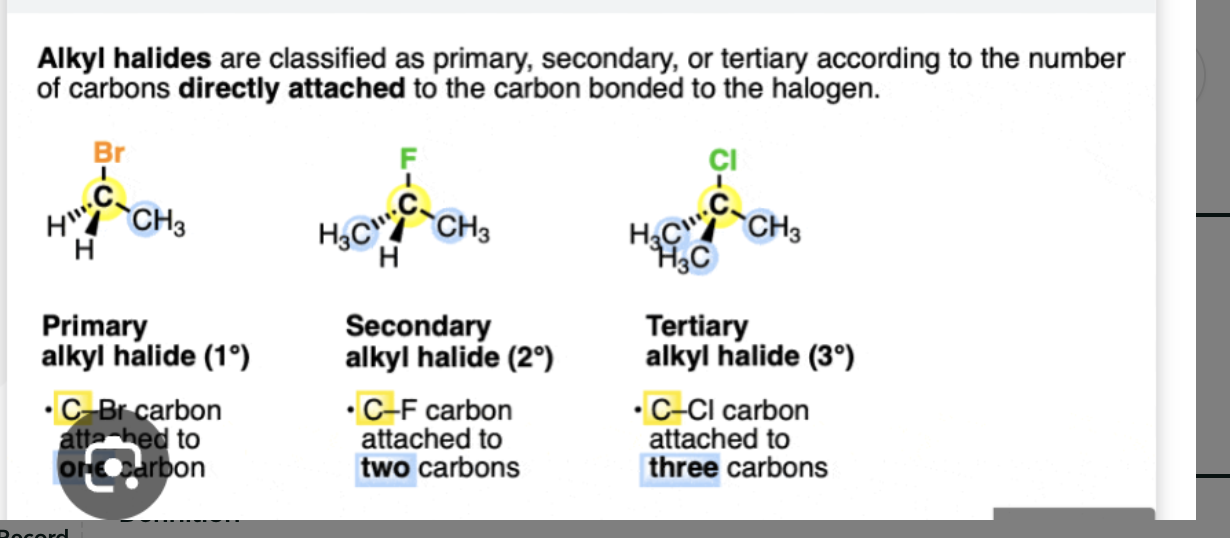
Primary, Secondary, or Tertiary halide
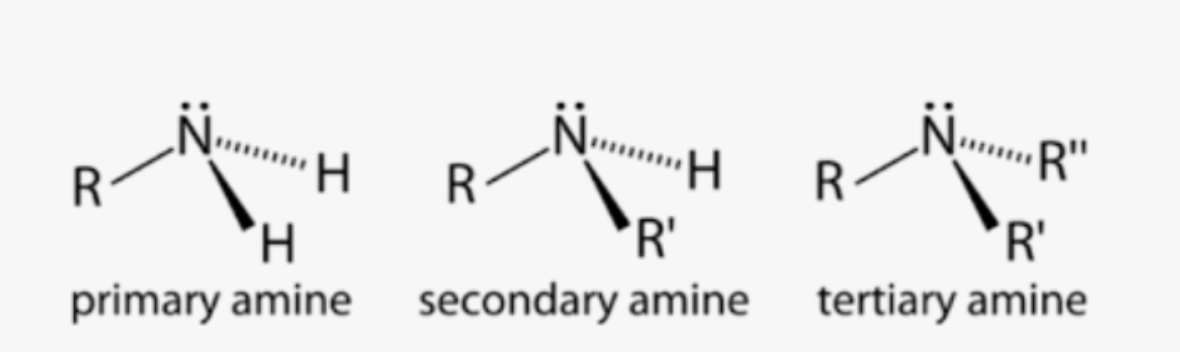
Primary, Secondary, or Tertiary amines
Determining higher BP
Ionic>H-bonding>Dipole-Dipole>LDF
SO FOR TWO COMPOUNDS WITH SIMILAR FUNCTIONAL GROUPS: the larger the surface area,/MORE SPREAD OUT, the HIGHER the boiling point, and the more polarizable the atoms, the higher the boiling point
A stronger IMF indicates a higher boiling point
Solubility
like dissolves like
H-Bonding
✅ H must be directly bonded to F, O, or N to donate a hydrogen bond.
✅ F, O, or N with lone pairs can accept hydrogen bonds.
To H bond to another molecule like itself, AND WATER, the molecule must contain an H bonded to O, N, or F
To H-bond with water, a molecule needs to contain only an O or N.
solubility
- More carbons mean LESS soluble
- <5 carbons = likely SOLUBLE in water
- >5 carbons = likely INSOLUBLE in water
Naming an Alcohol using the IUPAC system
1) Find the longest chain containing the carbon bonded to the OH group
2) Number the carbon chain to give the OH group the lower number, and apply all other rules of nomenclature
What counts when alphabetizing and naming
Prefixes that DON’T Count: di,tri,tetra, sec, tert, iso
Naming Alkenes
1) Find longest carbon chain
2) Number the chain so thatthe double bond gets the lowest possible number.
3) Put the number before the -ene to show where the double bond starts
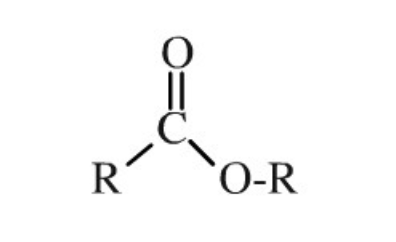
Ether

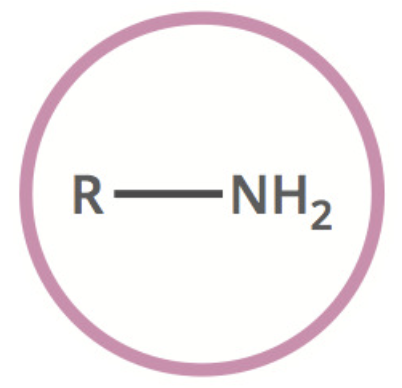
Amine
2 other types:
• R2-NH
• R3-N
Thiol
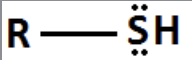
Sulfide
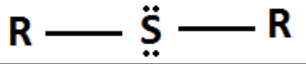
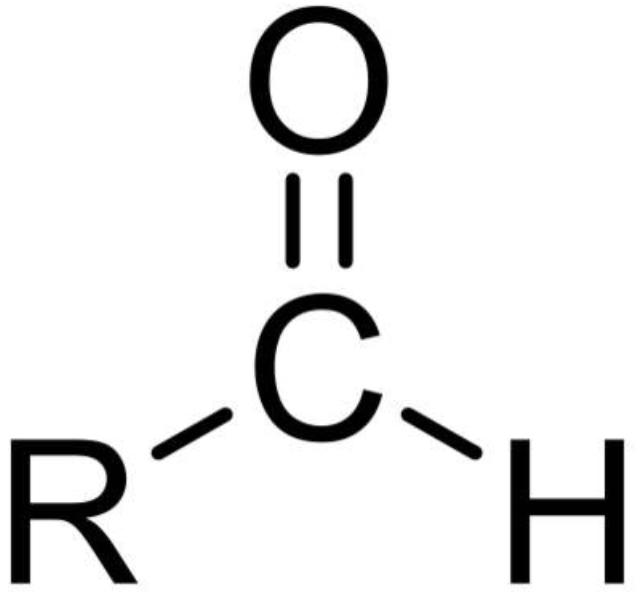
Aldehyde
Ketone
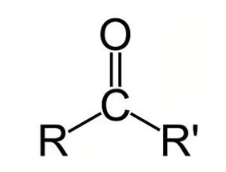
Carboxylic Acid
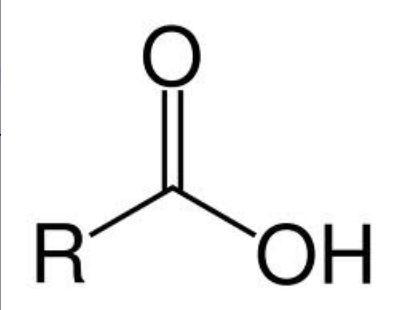
Ester
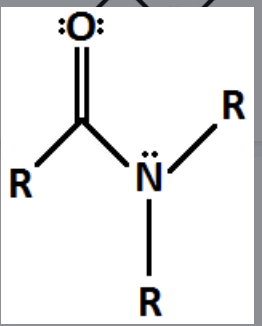
Amide
Chapter 3 - Structure: Constitutional, Stereochemical, and conformational Isomers
labeling stereogenic centers with R or S
1) identify the stereogenic center
2) assign priorities 1,2,3,4 (numbering associates with atomic # thus HIGHER NUMBER IMPLIES gets HIGHEST PRIORITY so 1 and usually hydrogen is 4 because it is the lightest
3)) IMPORTANT NOTE: LOWEST PRIORITY USUALLY or 4th priority but usually H and should be back dash. If it is showing up as a bolded dash switch the S to an R or vice versa.
Types of Conformations
Newman Projection: graphic that shows the 3 groups bonded to each carbon in a particular C-C bond. Eclipsed and Staggered are the two types of conformations.
Eclipsed vs Staggered Conformation
Eclipsed is less stable and more energy and staggered is more stable and less energy
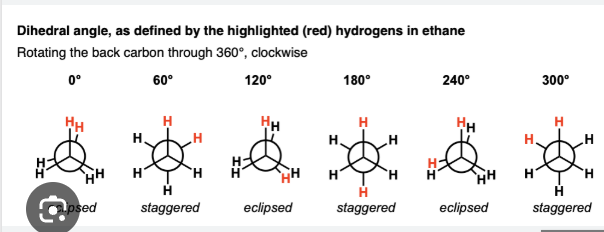
3 types of strains
1) Torsional
2) Steric
3) Angle
Ring strain
Refers to the instability in cyclic compounds due to angular distortion and torsional strain, often resulting in increased energy and reactivity. Ring strain is associated with bond angles INTERNAL to the ring
Torsional Strain
The strain that arises when atoms or groups of atoms are ECLIPSED or overlap in a molecule, causing increased energy and reduced stability.
Steric Strain
the interference between two bulky groups that are so close together that their electron clouds experience a repulsion causing increased energy and destabilization in the molecule.
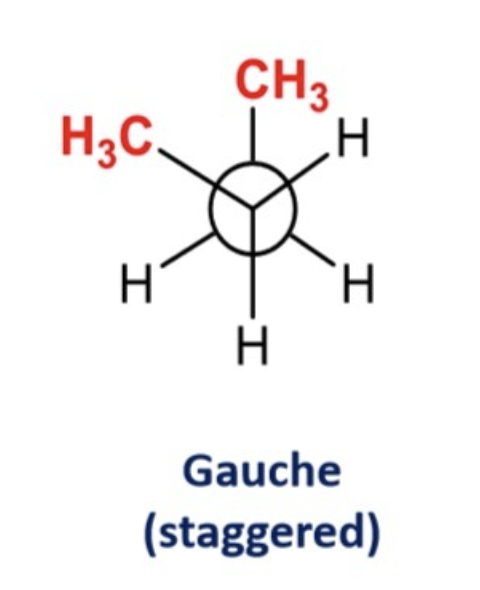
Angle Strain
It is more specific to cyclic compounds where the ring strucutre focuses bond angles away from the ideal tetrahedral. Puckering ring is when the ring bends and allows the molecule to adopt a more stable conformation by reducing unfavorable interactions
Angle Strain Trend
So, to clarify:
✅ Flat cyclohexane (theoretical) = 120° internal angles → more strain
✅ Chair conformation (real) = ~109.5° internal angles → less strain, more stable
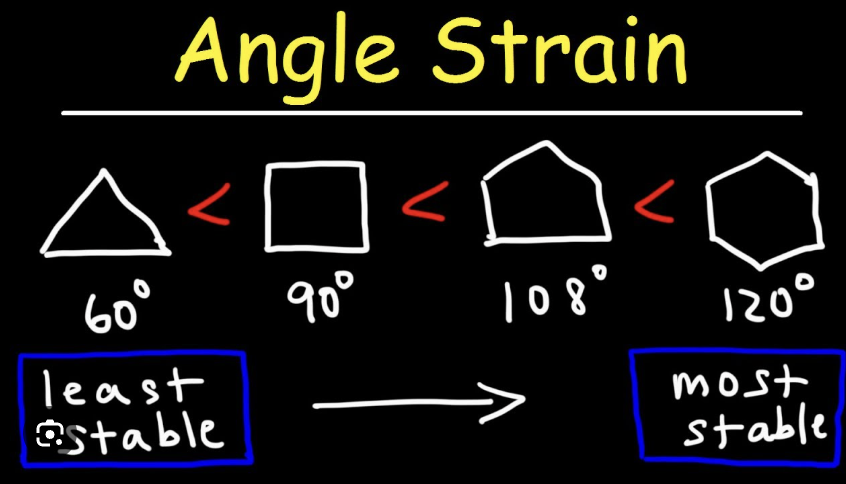
Two Types of Steric Strain
Anti and Guache
Gauche Interaction
- ALWAYS in a staggered conformation
- 2 groups NOT involving C-H
- the more gauche interactions the higher the energy thus less stable so will be more stable if newman projection has LESS GAUCHE INTERACTIONS
- bulky groups are 60 degrees apart
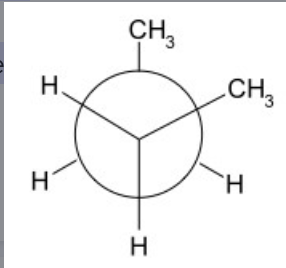
Anti Interaction
- ALWAYS in a staggered conformation
- bulky groups are 180 degrees apart thus reducing the steric strain thus is MORE STABLE than GAUCHE STAGGERED CONFORMATIONS
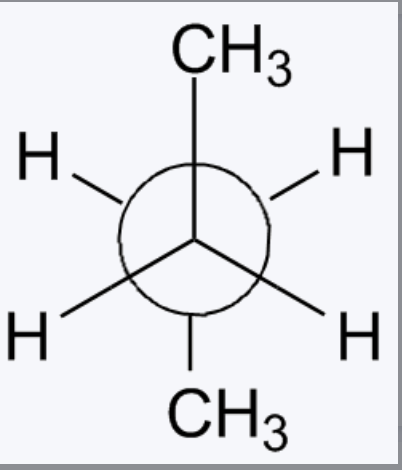
Fischer Projection
For two centers you do same horizontal are wedged.
look at page1 of ACS Final Notes
Chair Conformation
Ring Flipping
Equatorial is more stable than axial
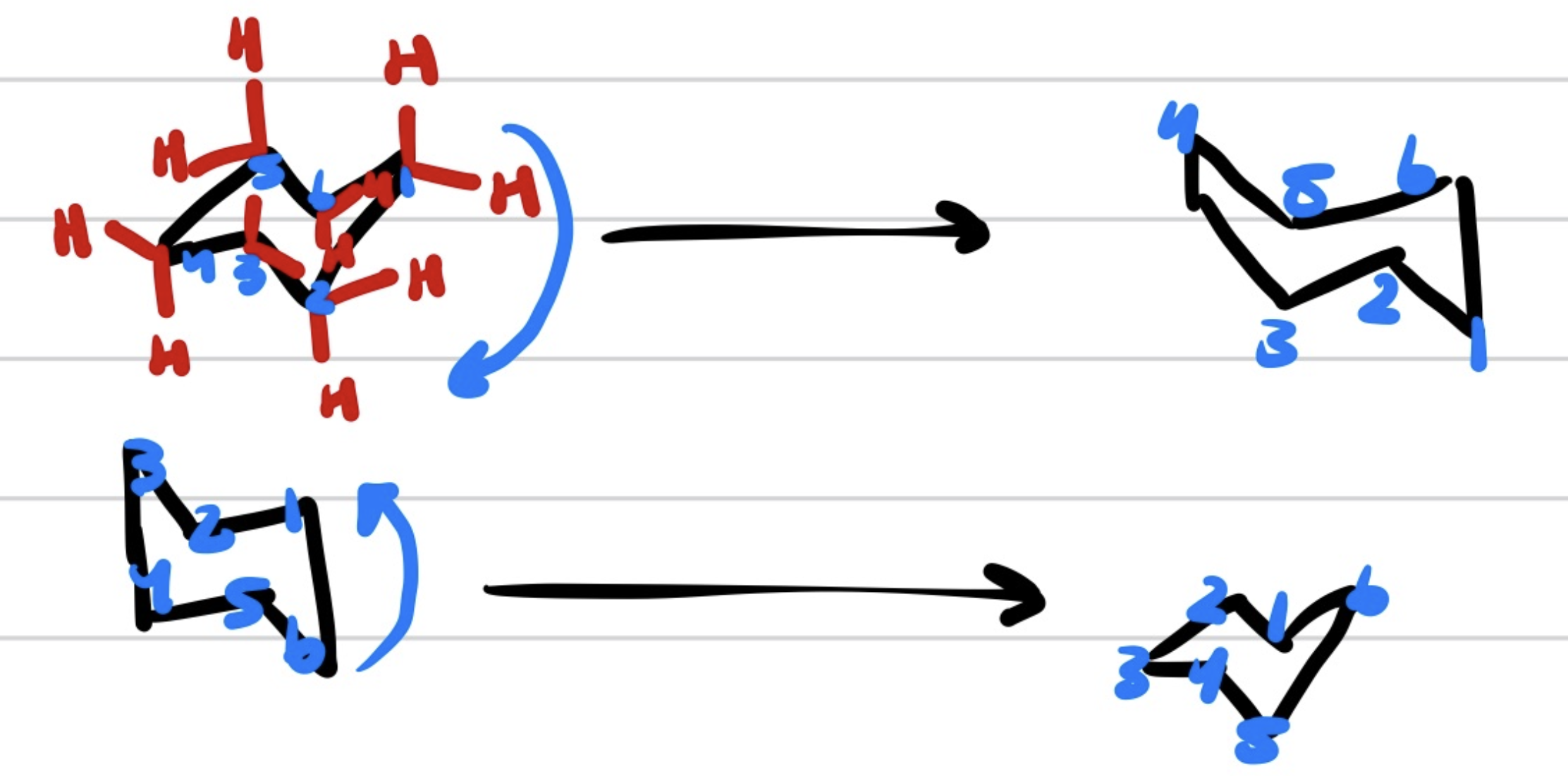
Up and Down Translates to
Up=wedged and Down=Dashed
Optical activity
Optically active compounds are those compounds that are chiral.
One enantiomer rotates plane-polarized light in clock wise dextrorotatory (+)
One enantiomer rotates in a plane-polarized in CC direction levorotatory (-) direction
Chiral Center
If there's only one chiral center, and the molecule has an internal plane of symmetry, then:
It's likely the chiral center is not truly chiral (the 4 groups aren’t actually different overall).
The molecule is achiral.
So, no enantiomer.
E and Z
Priority goes to higher atomic number attached to each side of double bond
Z = same
E=Different
Constitutional Vs Stereoisomers
Constitutional isomers have DIFFERENT connectivity of atoms and are ISOMERS, while stereoisomers differ in the spatial arrangement due to rotation around single bonds.
Enantiomers and diastereomers are types of stereoisomers
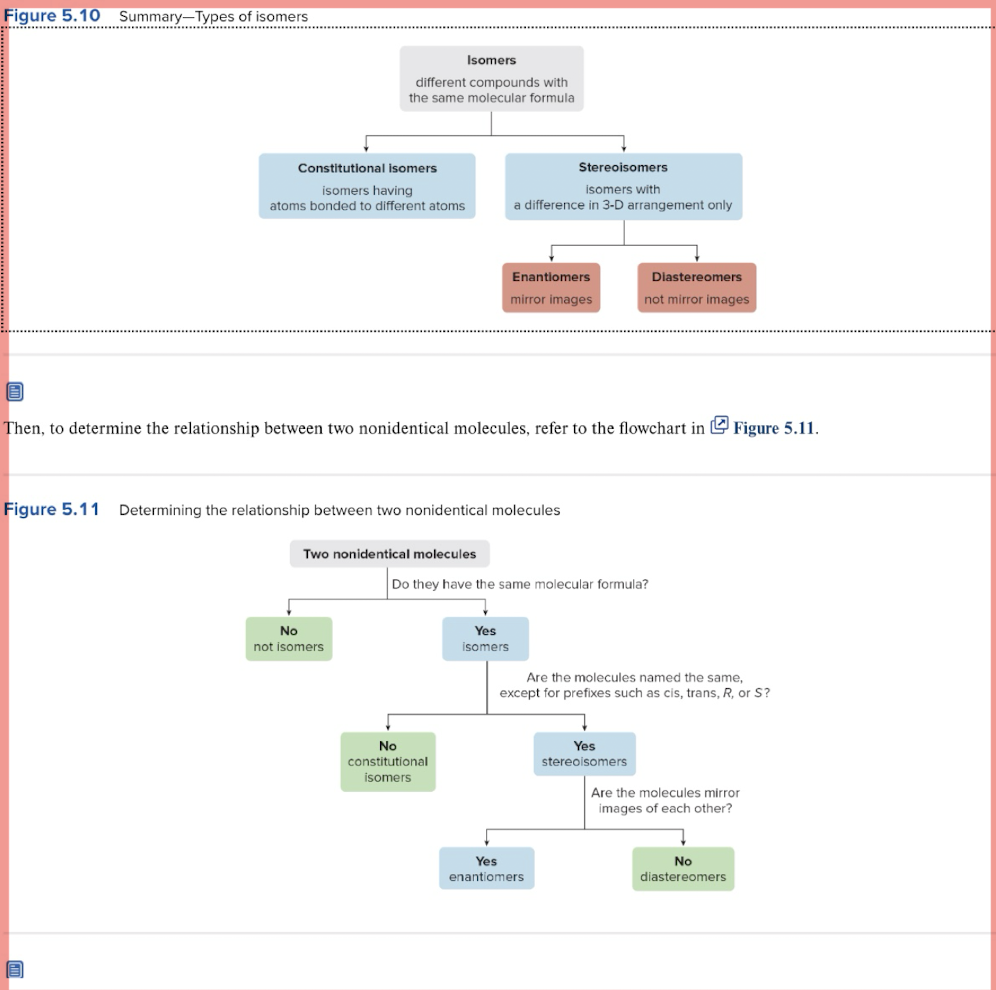
Meso Compound
Have at least two chiral centers
Have an internal plane of symmetry
MUST ALSO BE R and S CANT be RR or SS
But overall, the molecule is achiral
- ANOTHER RULE IS THE CARBON RULE where if even number of carbons between plane then should be DIFFERENT PLANE if odd number of carbons then should be SAME PLANE (exam 2 practice 24 for example)
Quick Tip for Enantiomers, Diastereomers, and Identical
💡 Quick tip:
Identical = exact same R/S on the exact same atoms (Ex: RS to RS)
Diastereomers = at least one R/S flipped, but not all
Enantiomers = all R/S flipped (mirror image)
Chapter 4: Acids and Bases
Bronsted Acid
A substance that donates a proton (H+) in a chemical reaction, thereby increasing the concentration of hydrogen ions in solution.
Bronsted Base
A substance that accepts a proton (H+) in a chemical reaction, decreasing the concentration of hydrogen ions in solution.
Lewis Acid
A substance that can accept an electron pair in a chemical reaction, often acting as an electrophile.
Lewis Base
A substance that can donate an electron pair in a chemical reaction, often acting as a nucleophile.
Nucleophiles
Electron rich, strong base
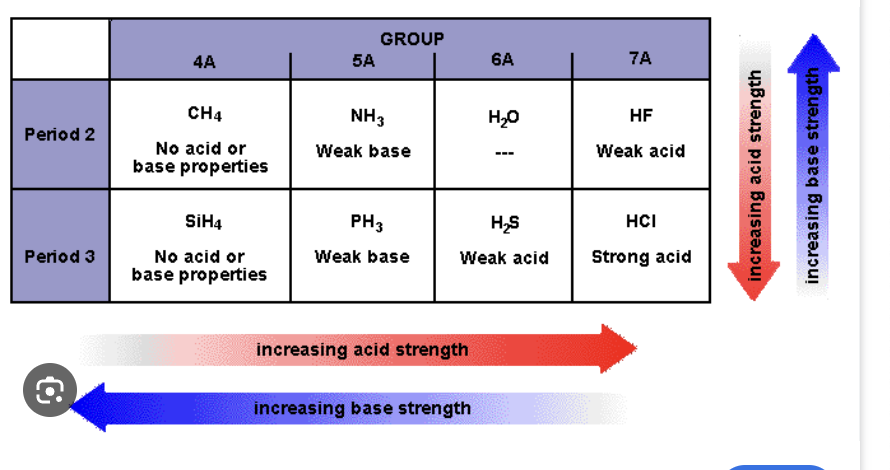
Acid and Base strength trend
K<1 vs K>1
K<1 is not spontaneous and favors reactants
K>1 is spontaneous and favors products
equilibrium favors formation of weaker acid/base
Basicity and Acidity Strength
- Element Effect
- Inductive effect
- Resonance effects
- Hybridization effects
Elemental Effect
Looking at trend of periodic table of acid and base strength
Inductive effect
- when an atom in a molecule has a strong inductive effect, meaning it pulls electrons towards itself, this stabilizes the conjugate base, making it weaker and therefore the corresponding acid stronger
Resonance Effect
More resonance structures for a molecule generally means increased acidity
Hybridization Effect
- higher % of s character of hybrid orbital the more stable the CB/weak thus stronger the acid
SP : 50% S character
SP2 : 33% S character
SP3: 25%
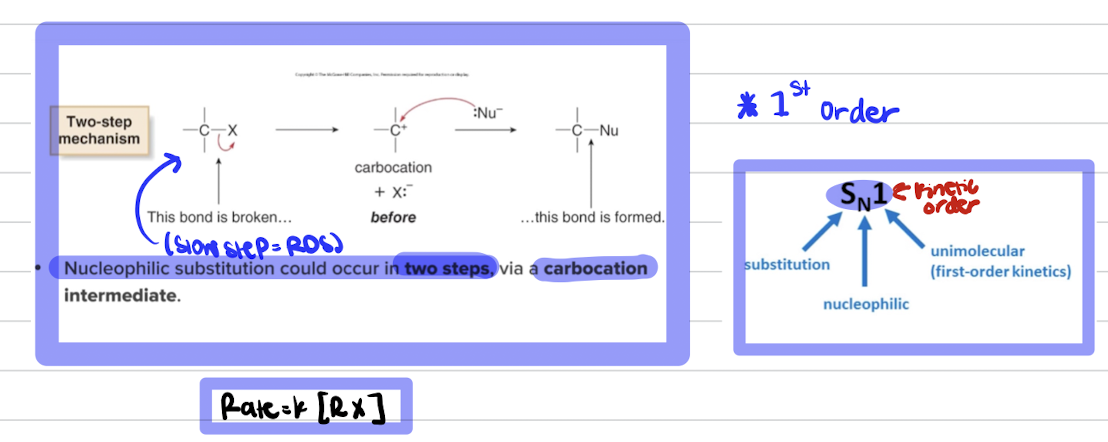
Chapter 5 - Nucleophilic Substitution Reactions
Rate Law Equations
Products NEVER appear in the rate law only reactants.
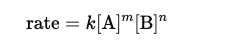
Rate Law equation for SN2
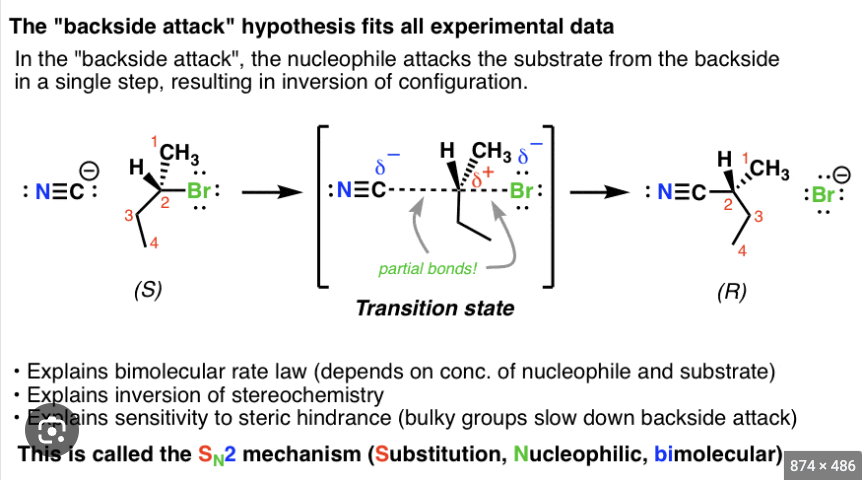
- 100% Inversion of configuration at Stereogenic Center meaning goes from R to S or S to R. CAN only do backside attack
SN2 Reactions do NOT produce enantiomer products instead they produce one SPECIFIC STEREOISOMER and its back side attack complete inversion
ONE-STEP and is a CONCERTED MECHANISM
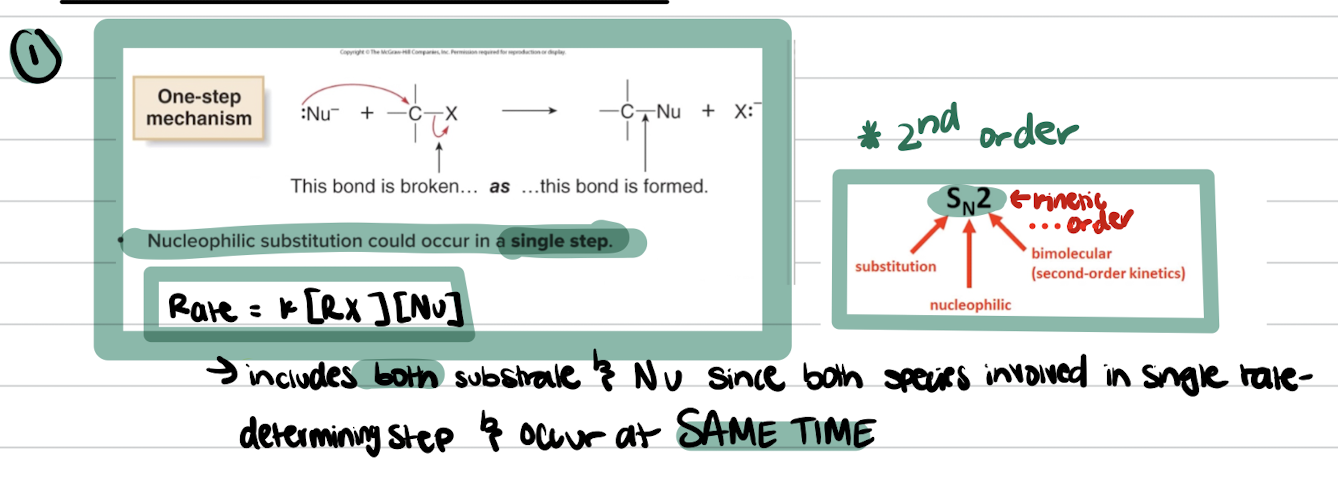
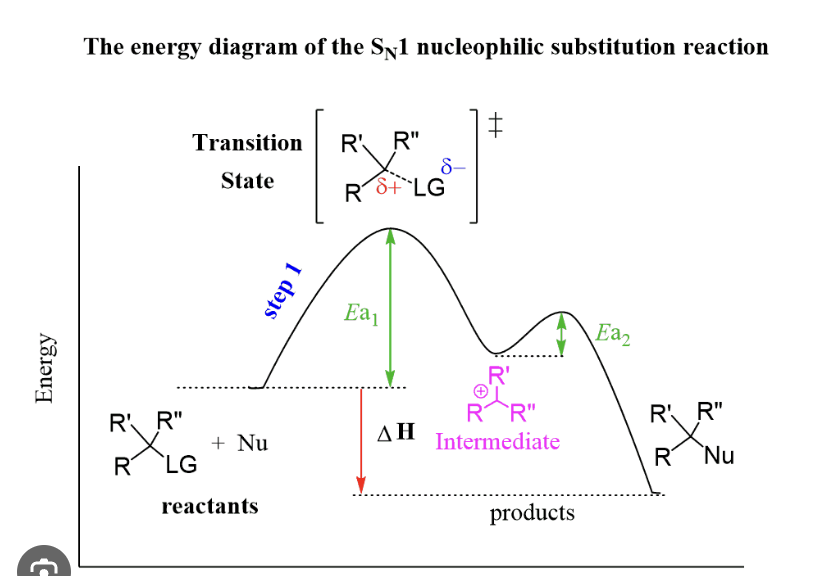
Rate Law equation for SN1
SN1 is a racemic mixture of products due to the formation of a planar carbocation intermediate, allowing for attack from either side. It involves a two-step mechanism.
Optical Rotation outcome for SN1 vs SN2
Optical rotation outcome for SN1:
If equal amounts of R and S enantiomers form, you get a racemic mixture.
A racemic mixture is optically inactive because the rotations cancel out.
[α]observed=0∘
Optical Rotation
For SN2, it is an inversion of stereochemistry, thus it gets opposite rotation angles
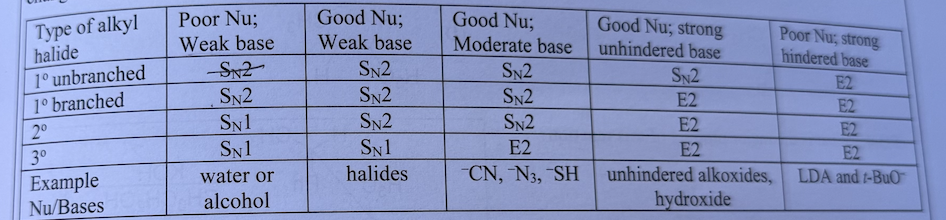
Polar Aprotic vs Polar Protic Solvents
Aprotic: Strong base is a strong NU, does not Hydrogen Bond, favored by SN2
Protic: Strong base and is a WEAK NU and does Hydrogen Bond favored by SN1
SN2 Summary
Methyl > 1º > 2º > 3º (increase rate of an Sn2)
- Primary is ALWAYS Sn2
- if given secondary look at what solvent aprotic or protic
- FAVORED BY STRONG Nu because relies on a DIRECT and CONCERTED attack occurs all in ONE STEP SO NEED IT TO BE STRONG
- STERIC HINDRANCE caused by bulky R groups makes hard for Nu to attack from back
- Sn2 has NO carbocation intermediates because occurs all in one step so no intermediates
- FAVORS Polar aprotic solvents
SN1 Summary
Methyl < 1º < 2º < 3º (increasing rate of an Sn1)
- faster when it is more substituted which means more stable carbocation
- tertiary is ALWAYS Sn2 because give stability to carbocation intermediate
- if given secondary look at what solvent aprotic or protic
- FAVORED BY WEAK Nu because Nu doesn't affect SN1 reaction rate as Nu ins't involved in the Rate determining step.
- Sn1 has carbocation intermediates because occurs in two steps
- FAVORS Polar protic solvents
Good Leaving Groups
Good Leaving groups are weak bases
Stability of Carbocation Intermediate
The stability of a carbocation determines the SN1 reaction rate, so the one that reacts the fastest is the most stable carbocation. Thus, the allylic carbocation or benzylic carbonation is most stable due to resonance, followed by tertiary, secondary, and primary carbocations.
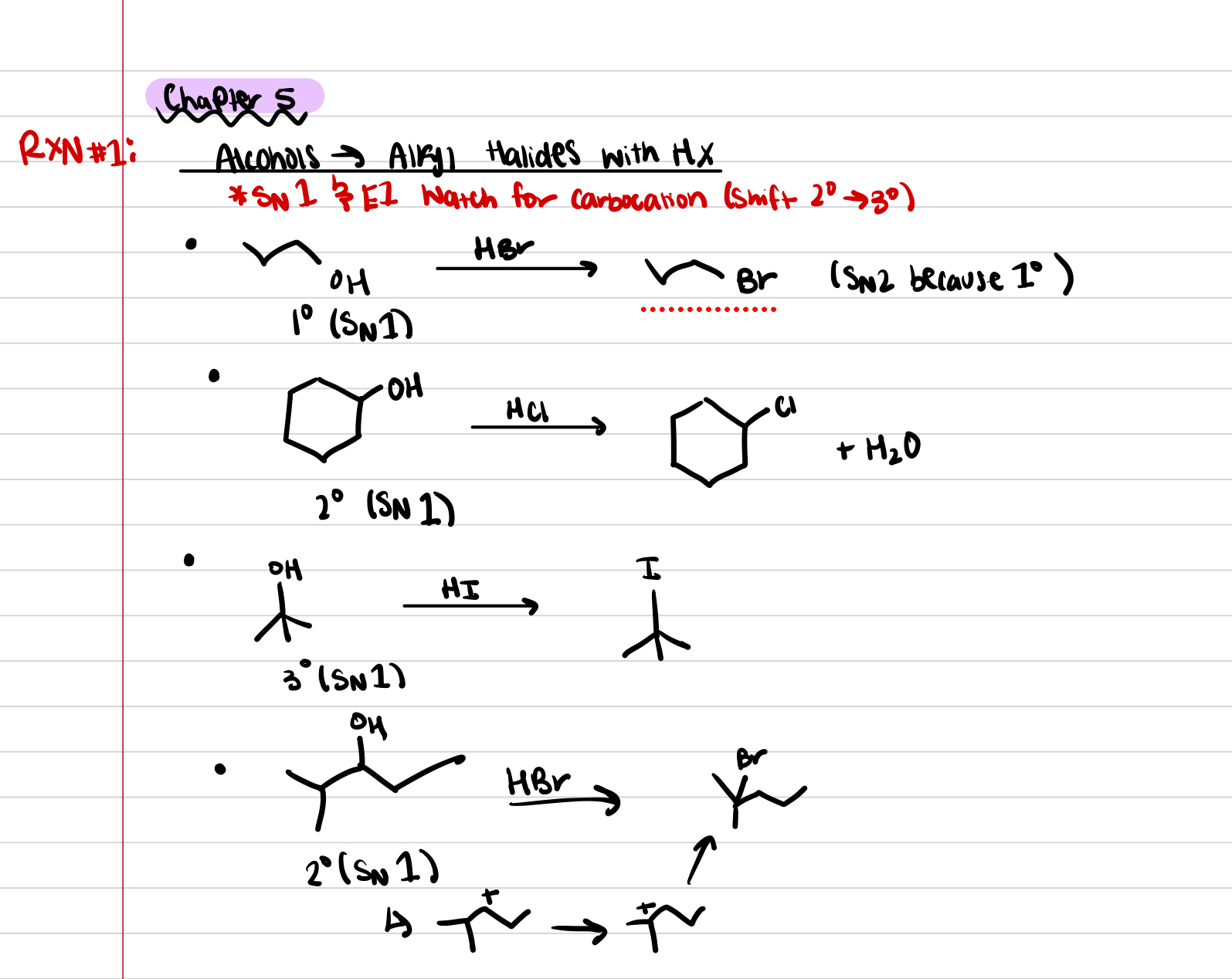
Reaction #1: Alcohols to Alkyl Halides with HX
Transition state for SN2
Transition state for SN1
Rate determining step is first step
in an SN1 reaction, the leaving group departs in the first step, and that step is the rate-determining step (RDS).
Breakdown:
SN1 = Substitution, Nucleophilic, Unimolecular
Step 1 (RDS): The leaving group leaves, forming a carbocation intermediate.
Step 2: The nucleophile attacks the carbocation to form the final product.
Acetone

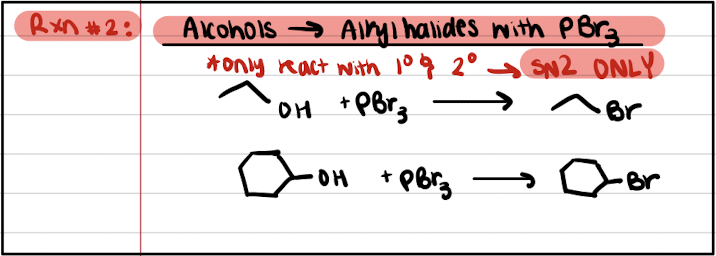
Reaction #2: Alcohols to Alkyl Halides with PBr3
Inversion of stereochem
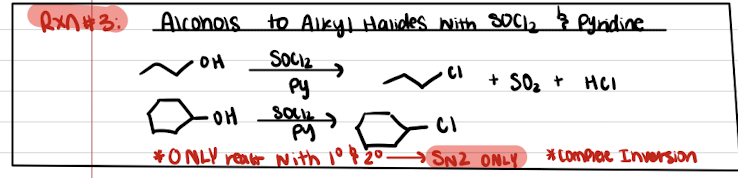
Reaction #3: Alcohols to Alkyl Halides with SOCl2 and Pyridine
Solvolysis
a nucleophilic substitution reaction where the solvent acts as the nucleophile.
haloacid
A haloacid is any halogen derivative of a carboxylic acid.
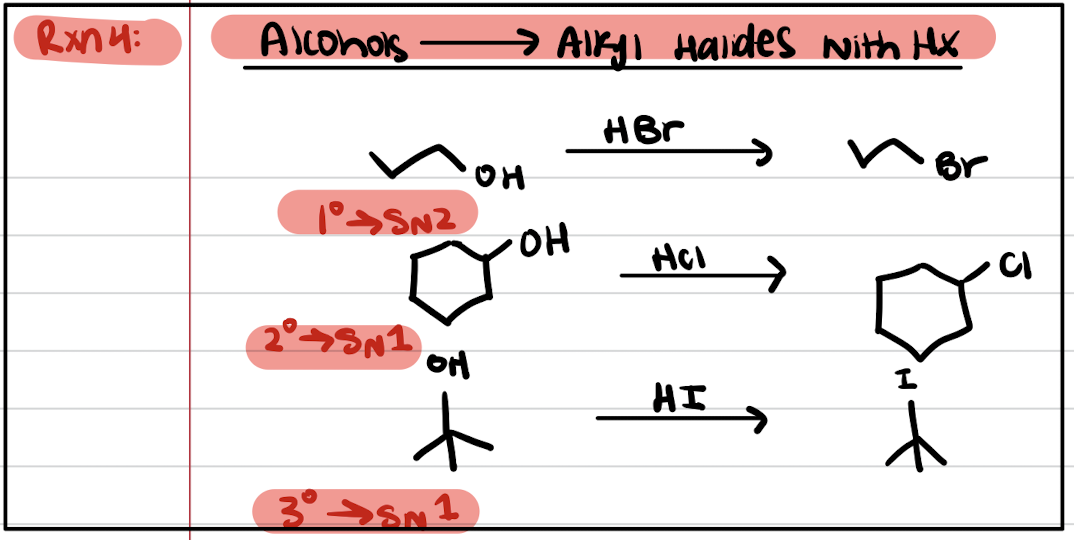
Reaction #4: Alcohols to Alkyl Halides with HX
FOR ALL SN1 and E1 reactions what to look out for?
LOOK OUT FOR METHYL AND HYDRIDE SHIFTS to create more stable carbocation
Ring Expansion SN1
Chapter 6 - Elimination Reactions
Break down of elimination Reactions
- identify beta carbon which is attached to hydrogen and identify alpha carbon which is attached to the halide.
1) remove the groups on the alpha and beta carbon (LG).
2) place a double bond between the the alpha and beta carbons
- NOTE that the base attacks the hydrogen on the beta carbon then the arrow goes to form double bond the arrow points to leaving group
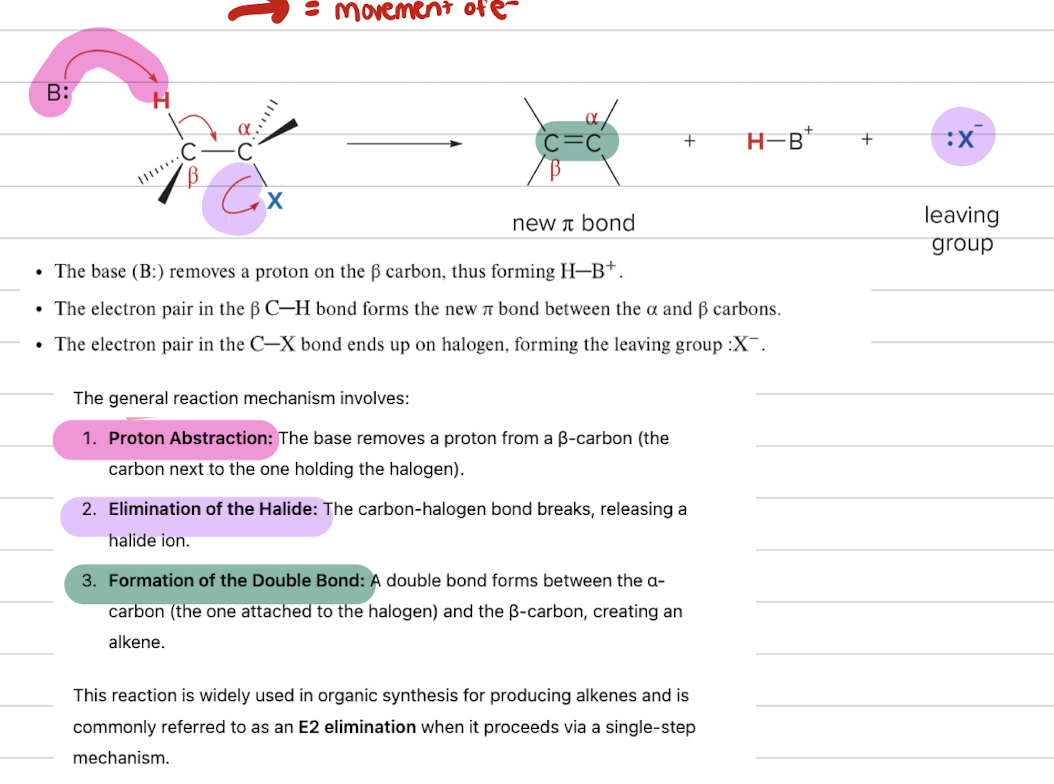
E2 Mechanism
- one step mechanism. bases attack same time as the leaving group leaves ALL BONDS ARE BROKEN AND FORMED AT SAME Time
- Bimolecular
- rate=k[Rx][Base]
- favors strong base because it will remove the beta hydrogen more easily
- favors polar aprotic solvent
- 2 and 3 alkyl halides
- E2 transition state questions look for all 4 partial bonds forming
Anti periplanar Conformation
- ONLY FOR E2 reactions
- antiperiplanar conformation is REQUIRED
- for cyclohexanes there exists 2 chair chofmrations
- NEED Leaving group in an AXIAL position in order for anti periplanar and for E2 Mech to occur the Beta hydrogen also needs to be in AXIAL
- have other bulky groups in EQUATORIAL POSITION
- redraw chair to cyclohexane to determine major or minor product
If not cyclohexane what is ideal position for elimination
The β-H and leaving group (on the α-carbon) must adopt a staggered conformation, where they are antiperiplanar — this is not the same as being trans in terms of stereochemistry.
Trans in this case = 180° dihedral angle
what is D?
D is a heavier isotope for Hydrogen\]
E1 Mechanism
- 2 step mechanism. leaving group leaves in first step creating the carbocation and the second step involves removal of the beta proton from the carbocation intermediate (slow step) from the base to produce alkene product.
- unimolecular
- rate=k[Rx]
- favors weak Nu and weak bases because the base doesn't appear in the rate equation
- favors polar protic solvent
- secondary and 3 alkyl halides. more substituents increases stability of the carbonation
DONT REACT WITH PRIMARY ALKYL HALIDES
![<p>- 2 step mechanism. leaving group leaves in first step creating the carbocation and the second step involves removal of the beta proton from the carbocation intermediate (slow step) from the base to produce alkene product.</p><p>- unimolecular</p><p>- rate=k[Rx]</p><p>- favors weak Nu and weak bases because the base doesn't appear in the rate equation</p><p>- favors polar protic solvent</p><p>- secondary and 3 alkyl halides. more substituents increases stability of the carbonation</p><p>DONT REACT WITH PRIMARY ALKYL HALIDES</p>](https://knowt-user-attachments.s3.amazonaws.com/65781710-4253-4703-8c45-71d18806236b.png)
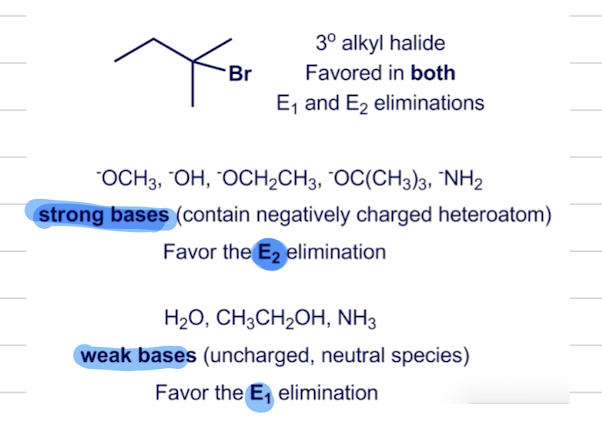
When is the Mechanism E1 or E2
Summary of Alkyl Halides and Sn1, Sn2, E1, and E2
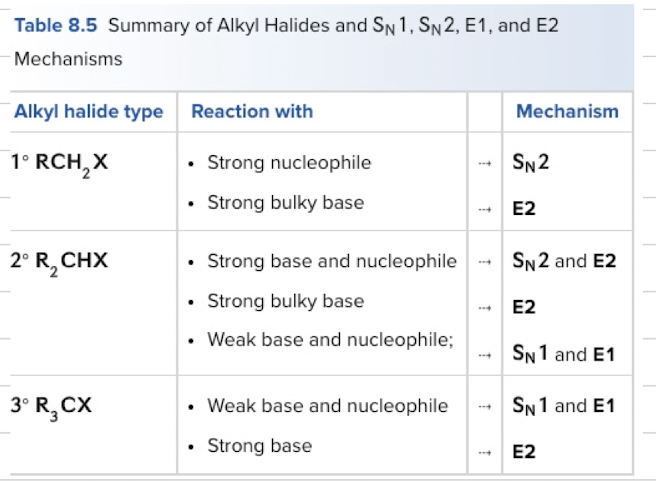
Summary of Alkyl Halides and Sn1, Sn2, E1, and E2
Determining Major Products in Elimination: Zaitsev’s and Hoffman Product
Zaitsev’s Rule:
more substituted product is the major product and is more stable, and use this for SMALL BASE
Hofmann Product:
For bulky bases least substituted alkene is favored
Reaction #5: Dehydration of Alchols to Alkenes

E1 and E2 which product is more favored (Substituted)
🧪 For Acid-Catalyzed Dehydration (E1 mechanism — most common for secondary/tertiary alcohols): ✅ More substituted alcohols are dehydrated faster.
Why? Because the rate-determining step is the formation of a carbocation.
More substituted carbocations (like tertiary) are more stable due to hyperconjugation and inductive effects.
Order of reactivity:
3° alcohol > 2° alcohol >> 1° alcohol
💡 Example:
tert-butanol (3°) loses water faster than ethanol (1°) under H₂SO₄/heat.
🧪 For E2 mechanism (usually with strong base and no carbocation): ✅ Less substituted alcohols may be faster if sterics are key (like with bulky bases).
E2 requires an anti-periplanar β-H and LG arrangement.
In this case, Hofmann product (less substituted alkene) may be favored if:
Bulky base (like t-BuOK)
Steric hindrance blocks more substituted elimination
But usually, Zaitsev’s product (more substituted alkene) is favored if there’s no hindrance.
What to look out for elimination product answers?
Choose trans productif applicable, consider steric factors.
Chapter 7 - Addition Reactions to Alkenes and Alkynes
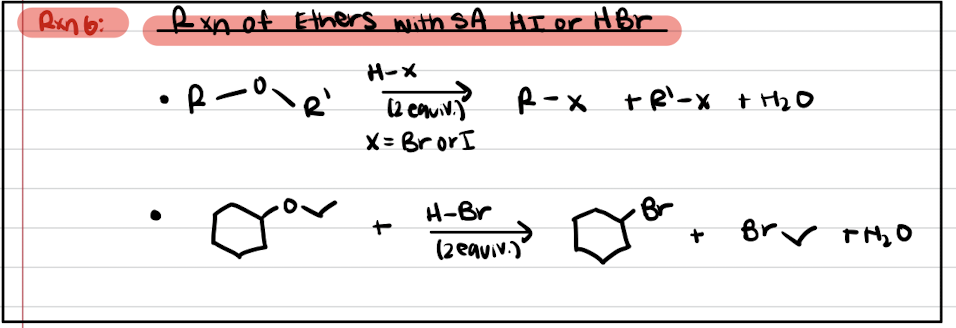
Reaction #6: Reaction of Ethers with SA HI or HBr